SUMOylation promotes PML degradation during encephalomyocarditis virus infection
- PMID: 20826694
- PMCID: PMC2977872
- DOI: 10.1128/JVI.01321-10
SUMOylation promotes PML degradation during encephalomyocarditis virus infection
Abstract
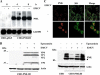
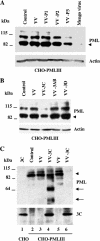
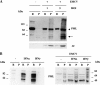
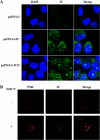
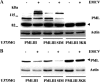
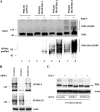
The promyelocytic leukemia (PML) protein is expressed in the diffuse nuclear fraction of the nucleoplasm and in matrix-associated structures, known as nuclear bodies (NBs). PML NB formation requires the covalent modification of PML to SUMO. The noncovalent interactions of SUMO with PML based on the identification of a SUMO-interacting motif within PML seem to be required for further recruitment within PML NBs of SUMOylated proteins. RNA viruses whose replication takes place in the cytoplasm and is inhibited by PML have developed various strategies to counteract the antiviral defense mediated by PML NBs. We show here that primary fibroblasts derived from PML knockout mice are more sensitive to infection with encephalomyocarditis virus (EMCV), suggesting that the absence of PML results in an increase in EMCV replication. Also, we found that EMCV induces a decrease in PML protein levels both in interferon-treated cells and in PMLIII-expressing cells. Reduction of PML was carried out by the EMCV 3C protease. Indeed, at early times postinfection, EMCV induced PML transfer from the nucleoplasm to the nuclear matrix and PML conjugation to SUMO-1, SUMO-2, and SUMO-3, leading to an increase in PML body size where the viral protease 3C and the proteasome component were found colocalizing with PML within the NBs. This process was followed by PML degradation occurring in a proteasome- and SUMO-dependent manner and did not involve the SUMO-interacting motif of PML. Together, these findings reveal a new mechanism evolved by EMCV to antagonize the PML pathway in the interferon-induced antiviral defense.
Figures



Similar articles
-
Promyelocytic leukemia isoform IV confers resistance to encephalomyocarditis virus via the sequestration of 3D polymerase in nuclear bodies.J Virol. 2011 Dec;85(24):13164-73. doi: 10.1128/JVI.05808-11. Epub 2011 Oct 12. J Virol. 2011. PMID: 21994459 Free PMC article.
-
Requirement of PML SUMO interacting motif for RNF4- or arsenic trioxide-induced degradation of nuclear PML isoforms.PLoS One. 2012;7(9):e44949. doi: 10.1371/journal.pone.0044949. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028697 Free PMC article.
-
Oxidative stress-induced assembly of PML nuclear bodies controls sumoylation of partner proteins.J Cell Biol. 2014 Mar 17;204(6):931-45. doi: 10.1083/jcb.201305148. J Cell Biol. 2014. PMID: 24637324 Free PMC article.
-
A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics.Int J Biol Sci. 2010 Jan 12;6(1):51-67. doi: 10.7150/ijbs.6.51. Int J Biol Sci. 2010. PMID: 20087442 Free PMC article. Review.
-
A Tale of Usurpation and Subversion: SUMO-Dependent Integrity of Promyelocytic Leukemia Nuclear Bodies at the Crossroad of Infection and Immunity.Front Cell Dev Biol. 2021 Aug 27;9:696234. doi: 10.3389/fcell.2021.696234. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513832 Free PMC article. Review.
Cited by
-
Desumoylation of RNA polymerase III lies at the core of the Sumo stress response in yeast.J Biol Chem. 2019 Dec 6;294(49):18784-18795. doi: 10.1074/jbc.RA119.009721. Epub 2019 Nov 1. J Biol Chem. 2019. PMID: 31676685 Free PMC article.
-
Nuclear remodelling during viral infections.Cell Microbiol. 2011 Jun;13(6):806-13. doi: 10.1111/j.1462-5822.2011.01596.x. Epub 2011 Apr 28. Cell Microbiol. 2011. PMID: 21501365 Free PMC article. Review.
-
Promyelocytic Leukemia Restricts Enterovirus 71 Replication by Inhibiting Autophagy.Front Immunol. 2018 Jun 5;9:1268. doi: 10.3389/fimmu.2018.01268. eCollection 2018. Front Immunol. 2018. PMID: 29922292 Free PMC article.
-
Viral evasion mechanisms of early antiviral responses involving regulation of ubiquitin pathways.Trends Microbiol. 2013 Aug;21(8):421-9. doi: 10.1016/j.tim.2013.06.006. Epub 2013 Jul 11. Trends Microbiol. 2013. PMID: 23850008 Free PMC article. Review.
-
Interplay between RNA Viruses and Promyelocytic Leukemia Nuclear Bodies.Vet Sci. 2021 Mar 31;8(4):57. doi: 10.3390/vetsci8040057. Vet Sci. 2021. PMID: 33807177 Free PMC article. Review.
References
-
- Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis viral protein 2A localizes to nucleoli and inhibits cap-dependent mRNA translation. Virus Res. 95:45-57. - PubMed
-
- Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59-73. - PubMed
-
- Amineva, S. P., A. G. Aminev, A. C. Palmenberg, and J. E. Gern. 2004. Rhinovirus 3C protease precursors 3CD and 3CD′ localize to the nuclei of infected cells. J. Gen. Virol. 85:2969-2979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

