microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7
- PMID: 20819939
- PMCID: PMC2935565
- DOI: 10.1083/jcb.200911036
microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7
Abstract
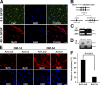
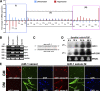
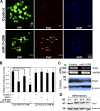
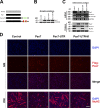
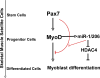
Skeletal muscle satellite cells are adult stem cells responsible for postnatal skeletal muscle growth and regeneration. Paired-box transcription factor Pax7 plays a central role in satellite cell survival, self-renewal, and proliferation. However, how Pax7 is regulated during the transition from proliferating satellite cells to differentiating myogenic progenitor cells is largely unknown. In this study, we find that miR-1 and miR-206 are sharply up-regulated during satellite cell differentiation and down-regulated after muscle injury. We show that miR-1 and miR-206 facilitate satellite cell differentiation by restricting their proliferative potential. We identify Pax7 as one of the direct regulatory targets of miR-1 and miR-206. Inhibition of miR-1 and miR-206 substantially enhances satellite cell proliferation and increases Pax7 protein level in vivo. Conversely, sustained Pax7 expression as a result of the loss of miR-1 and miR-206 repression elements at its 3' untranslated region significantly inhibits myoblast differentiation. Therefore, our experiments suggest that microRNAs participate in a regulatory circuit that allows rapid gene program transitions from proliferation to differentiation.
Figures


Similar articles
-
Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development.Nat Cell Biol. 2010 Mar;12(3):257-66. doi: 10.1038/ncb2025. Epub 2010 Jan 31. Nat Cell Biol. 2010. PMID: 20118923
-
Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression.Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13383-7. doi: 10.1073/pnas.0900210106. Epub 2009 Jul 28. Proc Natl Acad Sci U S A. 2009. PMID: 19666532 Free PMC article.
-
The role of microRNA-1 and microRNA-206 in the proliferation and differentiation of bovine skeletal muscle satellite cells.In Vitro Cell Dev Biol Anim. 2016 Jan;52(1):27-34. doi: 10.1007/s11626-015-9953-4. Epub 2015 Sep 30. In Vitro Cell Dev Biol Anim. 2016. PMID: 26424132
-
Satellite cells, the engines of muscle repair.Nat Rev Mol Cell Biol. 2011 Dec 21;13(2):127-33. doi: 10.1038/nrm3265. Nat Rev Mol Cell Biol. 2011. PMID: 22186952 Review.
-
Muscle stem cells in developmental and regenerative myogenesis.Curr Opin Clin Nutr Metab Care. 2010 May;13(3):243-8. doi: 10.1097/MCO.0b013e328336ea98. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20098319 Free PMC article. Review.
Cited by
-
Role of exosomes and exosomal microRNA in muscle-Kidney crosstalk in chronic kidney disease.Front Cell Dev Biol. 2022 Sep 7;10:951837. doi: 10.3389/fcell.2022.951837. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158193 Free PMC article. Review.
-
MicroRNA-206 regulates cell movements during zebrafish gastrulation by targeting prickle1a and regulating c-Jun N-terminal kinase 2 phosphorylation.Mol Cell Biol. 2012 Jul;32(14):2934-42. doi: 10.1128/MCB.00134-12. Epub 2012 May 21. Mol Cell Biol. 2012. PMID: 22615492 Free PMC article.
-
Functional and Morphological Improvement of Dystrophic Muscle by Interleukin 6 Receptor Blockade.EBioMedicine. 2015 Feb 26;2(4):285-93. doi: 10.1016/j.ebiom.2015.02.014. eCollection 2015 Apr. EBioMedicine. 2015. PMID: 26137572 Free PMC article.
-
MicroRNA-431 accelerates muscle regeneration and ameliorates muscular dystrophy by targeting Pax7 in mice.Nat Commun. 2015 Jul 7;6:7713. doi: 10.1038/ncomms8713. Nat Commun. 2015. PMID: 26151913
-
Circulating MicroRNAs as Potential Biomarkers of Exercise Response.Int J Mol Sci. 2016 Oct 5;17(10):1553. doi: 10.3390/ijms17101553. Int J Mol Sci. 2016. PMID: 27782053 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

