The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis
- PMID: 20807880
- PMCID: PMC2947187
- DOI: 10.1105/tpc.109.072215
The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis
Abstract
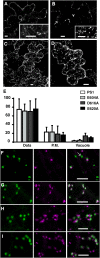
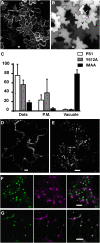
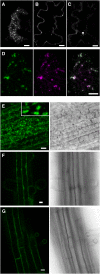
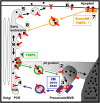
Pea (Pisum sativum) BP80 is a vacuolar sorting receptor for soluble proteins and has a cytosolic domain essential for its intracellular trafficking between the trans-Golgi network and the prevacuole. Based on mammalian knowledge, we introduced point mutations in the cytosolic region of the receptor and produced chimeras of green fluorescent protein fused to the transmembrane domain of pea BP80 along with the modified cytosolic tails. By analyzing the subcellular location of these chimera, we found that mutating Glu-604, Asp-616, or Glu-620 had mild effects, whereas mutating the Tyr motif partially redistributed the chimera to the plasma membrane. Replacing both Ile-608 and Met-609 by Ala (IMAA) led to a massive redistribution of fluorescence to the vacuole, indicating that recycling is impaired. When the chimera uses the alternative route, the IMAA mutation led to a massive accumulation at the plasma membrane. Using Arabidopsis thaliana plants expressing a fluorescent reporter with the full-length sequence of At VSR4, we demonstrated that the receptor undergoes brefeldin A-sensitive endocytosis. We conclude that the receptors use two pathways, one leading directly to the lytic vacuole and the other going via the plasma membrane, and that the Ileu-608 Met-609 motif has a role in the retrieval step in both pathways.
Figures



Similar articles
-
Nanobody-triggered lockdown of VSRs reveals ligand reloading in the Golgi.Nat Commun. 2018 Feb 13;9(1):643. doi: 10.1038/s41467-018-02909-6. Nat Commun. 2018. PMID: 29440677 Free PMC article.
-
Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail.Plant Cell. 2006 Jun;18(6):1477-97. doi: 10.1105/tpc.105.040394. Epub 2006 May 19. Plant Cell. 2006. PMID: 16714388 Free PMC article.
-
Molecular cloning and further characterization of a probable plant vacuolar sorting receptor.Plant Physiol. 1997 Sep;115(1):29-39. doi: 10.1104/pp.115.1.29. Plant Physiol. 1997. PMID: 9306690 Free PMC article.
-
Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization.FEBS J. 2011 Jan;278(1):59-68. doi: 10.1111/j.1742-4658.2010.07923.x. Epub 2010 Nov 16. FEBS J. 2011. PMID: 21078125 Review.
-
Protein sorting to the storage vacuoles of plants: a critical appraisal.Traffic. 2005 Aug;6(8):615-25. doi: 10.1111/j.1600-0854.2005.00303.x. Traffic. 2005. PMID: 15998318 Review.
Cited by
-
pH Regulation by NHX-Type Antiporters Is Required for Receptor-Mediated Protein Trafficking to the Vacuole in Arabidopsis.Plant Cell. 2015 Apr;27(4):1200-17. doi: 10.1105/tpc.114.135699. Epub 2015 Mar 31. Plant Cell. 2015. PMID: 25829439 Free PMC article.
-
Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis.Plant Cell. 2011 Sep;23(9):3463-81. doi: 10.1105/tpc.111.086918. Epub 2011 Sep 20. Plant Cell. 2011. PMID: 21934143 Free PMC article.
-
Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis.Cell Res. 2012 Feb;22(2):413-24. doi: 10.1038/cr.2011.129. Epub 2011 Aug 9. Cell Res. 2012. PMID: 21826108 Free PMC article.
-
Receptor-mediated transport of vacuolar proteins: a critical analysis and a new model.Protoplasma. 2014 Jan;251(1):247-64. doi: 10.1007/s00709-013-0542-7. Epub 2013 Sep 10. Protoplasma. 2014. PMID: 24019013
-
Nanobody-triggered lockdown of VSRs reveals ligand reloading in the Golgi.Nat Commun. 2018 Feb 13;9(1):643. doi: 10.1038/s41467-018-02909-6. Nat Commun. 2018. PMID: 29440677 Free PMC article.
References
-
- Blom N., Gammeltoft S., Brunak S. (1999). Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294: 1351–1362 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

