Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth
- PMID: 20807879
- PMCID: PMC2947167
- DOI: 10.1105/tpc.110.076257
Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth
Abstract
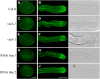
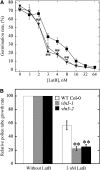
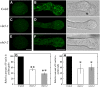
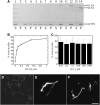
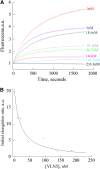
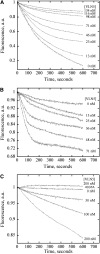
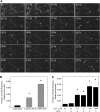
A dynamic actin cytoskeleton is essential for pollen germination and tube growth. However, the molecular mechanisms underlying the organization and turnover of the actin cytoskeleton in pollen remain poorly understood. Villin plays a key role in the formation of higher-order structures from actin filaments and in the regulation of actin dynamics in eukaryotic cells. It belongs to the villin/gelsolin/fragmin superfamily of actin binding proteins and is composed of six gelsolin-homology domains at its core and a villin headpiece domain at its C terminus. Recently, several villin family members from plants have been shown to sever, cap, and bundle actin filaments in vitro. Here, we characterized a villin isovariant, Arabidopsis thaliana VILLIN5 (VLN5), that is highly and preferentially expressed in pollen. VLN5 loss-of-function retarded pollen tube growth and sensitized actin filaments in pollen grains and tubes to latrunculin B. In vitro biochemical analyses revealed that VLN5 is a typical member of the villin family and retains a full suite of activities, including barbed-end capping, filament bundling, and calcium-dependent severing. The severing activity was confirmed with time-lapse evanescent wave microscopy of individual actin filaments in vitro. We propose that VLN5 is a major regulator of actin filament stability and turnover that functions in concert with oscillatory calcium gradients in pollen and therefore plays an integral role in pollen germination and tube growth.
Figures




Similar articles
-
Arabidopsis villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars.Plant Cell. 2013 May;25(5):1803-17. doi: 10.1105/tpc.113.110940. Epub 2013 May 28. Plant Cell. 2013. PMID: 23715472 Free PMC article.
-
Arabidopsis VILLIN5 bundles actin filaments using a novel mechanism.Plant J. 2024 Sep;119(6):2854-2866. doi: 10.1111/tpj.16956. Epub 2024 Aug 2. Plant J. 2024. PMID: 39093617
-
Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization.Plant Cell. 2005 Feb;17(2):486-501. doi: 10.1105/tpc.104.028555. Epub 2005 Jan 19. Plant Cell. 2005. PMID: 15659626 Free PMC article.
-
Plant villins: versatile actin regulatory proteins.J Integr Plant Biol. 2015 Jan;57(1):40-9. doi: 10.1111/jipb.12293. Epub 2014 Dec 17. J Integr Plant Biol. 2015. PMID: 25294278 Review.
-
Actin dynamics in the cortical array of plant cells.Curr Opin Plant Biol. 2013 Dec;16(6):678-87. doi: 10.1016/j.pbi.2013.10.012. Epub 2013 Nov 15. Curr Opin Plant Biol. 2013. PMID: 24246228 Review.
Cited by
-
Visualization of Actin Organization and Quantification in Fixed Arabidopsis Pollen Grains and Tubes.Bio Protoc. 2020 Jan 5;10(1):e3509. doi: 10.21769/BioProtoc.3509. eCollection 2020 Jan 5. Bio Protoc. 2020. PMID: 33654717 Free PMC article.
-
Control of cell wall extensibility during pollen tube growth.Mol Plant. 2013 Jul;6(4):998-1017. doi: 10.1093/mp/sst103. Epub 2013 Jun 14. Mol Plant. 2013. PMID: 23770837 Free PMC article. Review.
-
VILLIN2 regulates cotton defense against Verticillium dahliae by modulating actin cytoskeleton remodeling.Plant Physiol. 2023 May 2;192(1):666-679. doi: 10.1093/plphys/kiad095. Plant Physiol. 2023. PMID: 36881883 Free PMC article.
-
Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20.Plant Cell. 2013 Nov;25(11):4525-43. doi: 10.1105/tpc.113.118463. Epub 2013 Nov 26. Plant Cell. 2013. PMID: 24280384 Free PMC article.
-
Gelsolin-Like Domain 3 Plays Vital Roles in Regulating the Activities of the Lily Villin/Gelsolin/Fragmin Superfamily.PLoS One. 2015 Nov 20;10(11):e0143174. doi: 10.1371/journal.pone.0143174. eCollection 2015. PLoS One. 2015. PMID: 26587673 Free PMC article.
References
-
- Andrianantoandro E., Pollard T.D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24: 13–23 - PubMed
-
- Blanchoin L., Amann K.J., Higgs H.N., Marchand J.B., Kaiser D.A., Pollard T.D. (2000). Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404: 1007–1011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

