Immature NK cells, capable of producing IL-22, are present in human uterine mucosa
- PMID: 20802153
- PMCID: PMC3795409
- DOI: 10.4049/jimmunol.1001637
Immature NK cells, capable of producing IL-22, are present in human uterine mucosa
Abstract
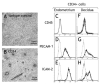
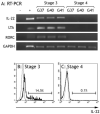
NK cells are the dominant population of immune cells in the endometrium in the secretory phase of the menstrual cycle and in the decidua in early pregnancy. The possibility that this is a site of NK cell development is of particular interest because of the cyclical death and regeneration of the NK population during the menstrual cycle. To investigate this, we searched for NK developmental stages 1-4, based on expression of CD34, CD117, and CD94. In this study, we report that a heterogeneous population of stage 3 NK precursor (CD34(-)CD117(+)CD94(-)) and mature stage 4 NK (CD34(-)CD117(-/+)CD94(+)) cells, but not multipotent stages 1 and 2 (CD34(+)), are present in the uterine mucosa. Cells within the uterine stage 3 population are able to give rise to mature stage 4-like cells in vitro but also produce IL-22 and express RORC and LTA. We also found stage 3 cells with NK progenitor potential in peripheral blood. We propose that stage 3 cells are recruited from the blood to the uterus and mature in the uterine microenvironment to become distinctive uterine NK cells. IL-22 producers in this population might have a physiological role in this specialist mucosa dedicated to reproduction.
Figures



Similar articles
-
Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue.Immunity. 2010 Jun 25;32(6):803-14. doi: 10.1016/j.immuni.2010.06.007. Immunity. 2010. PMID: 20620944 Free PMC article.
-
The effect of pregnancy on the uterine NK cell KIR repertoire.Eur J Immunol. 2011 Oct;41(10):3017-27. doi: 10.1002/eji.201141445. Epub 2011 Aug 30. Eur J Immunol. 2011. PMID: 21739430 Free PMC article.
-
Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22.Blood. 2009 Apr 23;113(17):4008-10. doi: 10.1182/blood-2008-12-192443. Epub 2009 Feb 24. Blood. 2009. PMID: 19244159 Free PMC article.
-
The unique properties of human NK cells in the uterine mucosa.Placenta. 2008 Mar;29 Suppl A:S60-6. doi: 10.1016/j.placenta.2007.10.006. Epub 2007 Nov 26. Placenta. 2008. PMID: 18039547 Review.
-
Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus.J Reprod Immunol. 2003 Aug;59(2):175-91. doi: 10.1016/s0165-0378(03)00046-9. J Reprod Immunol. 2003. PMID: 12896821 Review.
Cited by
-
Emerging role of C5aR2: novel insights into the regulation of uterine immune cells during pregnancy.Front Immunol. 2024 Jun 20;15:1411315. doi: 10.3389/fimmu.2024.1411315. eCollection 2024. Front Immunol. 2024. PMID: 38979410 Free PMC article.
-
IL-22 Plays a Dual Role in the Amniotic Cavity: Tissue Injury and Host Defense against Microbes in Preterm Labor.J Immunol. 2022 Apr 1;208(7):1595-1615. doi: 10.4049/jimmunol.2100439. Epub 2022 Mar 18. J Immunol. 2022. PMID: 35304419 Free PMC article.
-
Natural killer (NK) and NK-like cells at mucosal epithelia: Mediators of anti-microbial defense and maintenance of tissue integrity.Eur J Microbiol Immunol (Bp). 2011 Dec;1(4):257-66. doi: 10.1556/EuJMI.1.2011.4.1. Epub 2011 Dec 23. Eur J Microbiol Immunol (Bp). 2011. PMID: 24516732 Free PMC article. Review.
-
NK cell receptor profiling of endometrial and decidual NK cells reveals pregnancy-induced adaptations.Front Immunol. 2024 Mar 20;15:1353556. doi: 10.3389/fimmu.2024.1353556. eCollection 2024. Front Immunol. 2024. PMID: 38571943 Free PMC article.
-
Exploring the NK cell platform for cancer immunotherapy.Nat Rev Clin Oncol. 2021 Feb;18(2):85-100. doi: 10.1038/s41571-020-0426-7. Epub 2020 Sep 15. Nat Rev Clin Oncol. 2021. PMID: 32934330 Free PMC article. Review.
References
-
- Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 2006;24:257–86. - PubMed
-
- Haller O, Wigzell H. Suppression of natural killer cell activity with radioactive strontium: effector cells are marrow dependent. J. Immunol. 1977;118:1503–6. - PubMed
-
- Kumar V, Ben-Ezra J, Bennett M, Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J. Immunol. 1979;123:1832–8. - PubMed
-
- Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 2001;31:1900–9. - PubMed
-
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le-Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 2006;7:1217–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

