Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway
- PMID: 20802151
- PMCID: PMC2988655
- DOI: 10.4049/jimmunol.1000801
Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway
Abstract
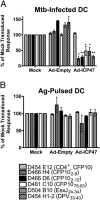
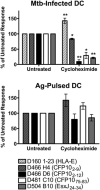
Exposure to Mycobacterium tuberculosis can result in lifelong but asymptomatic infection in most individuals. Although CD8(+) T cells are elicited at high frequencies over the course of infection in both humans and mice, how phagosomal M. tuberculosis Ags are processed and presented by MHC class I molecules is poorly understood. Broadly, both cytosolic and noncytosolic pathways have been described. We have previously characterized the presentation of three HLA-I epitopes from M. tuberculosis and shown that these Ags are processed in the cytosol, whereas others have demonstrated noncytosolic presentation of the 19-kDa lipoprotein as well as apoptotic bodies from M. tuberculosis-infected cells. In this paper, we now characterize the processing pathway in an additional six M. tuberculosis epitopes from four proteins in human dendritic cells. Addition of the endoplasmic reticulum-Golgi trafficking inhibitor, brefeldin A, resulted in complete abrogation of Ag processing consistent with cytosolic presentation. However, although addition of the proteasome inhibitor epoxomicin blocked the presentation of two epitopes, presentation of four epitopes was enhanced. To further examine the requirement for proteasomal processing of an epoxomicin-enhanced epitope, an in vitro proteasome digestion assay was established. We find that the proteasome does indeed generate the epitope and that epitope generation is enhanced in the presence of epoxomicin. To further confirm that both the epoxomicin-inhibited and epoxomicin-enhanced epitopes are processed cytosolically, we demonstrate that TAP transport and new protein synthesis are required for presentation. Taken together, these data demonstrate that immunodominant M. tuberculosis CD8(+) Ags are processed and presented using a cytosolic pathway.
Figures
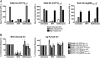
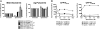

Similar articles
-
Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages.J Immunol. 2004 May 1;172(9):5277-86. doi: 10.4049/jimmunol.172.9.5277. J Immunol. 2004. PMID: 15100266
-
Efficient delivery of Antennapedia homeodomain fused to CTL epitope with liposomes into dendritic cells results in the activation of CD8+ T cells.J Immunol. 2001 Dec 1;167(11):6462-70. doi: 10.4049/jimmunol.167.11.6462. J Immunol. 2001. PMID: 11714813
-
The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle.PLoS Pathog. 2009 Apr;5(4):e1000374. doi: 10.1371/journal.ppat.1000374. Epub 2009 Apr 10. PLoS Pathog. 2009. PMID: 19360129 Free PMC article.
-
MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses.Immunol Rev. 1997 Aug;158:129-36. doi: 10.1111/j.1600-065x.1997.tb00999.x. Immunol Rev. 1997. PMID: 9314081 Review.
-
Involvement of autophagy in MHC class I antigen presentation.Scand J Immunol. 2020 Nov;92(5):e12978. doi: 10.1111/sji.12978. Epub 2020 Oct 19. Scand J Immunol. 2020. PMID: 32969499 Free PMC article. Review.
Cited by
-
The Goldilocks model of immune symbiosis with Mycobacteria and Candida colonizers.Cytokine. 2017 Sep;97:49-65. doi: 10.1016/j.cyto.2017.05.015. Epub 2017 May 29. Cytokine. 2017. PMID: 28570933 Free PMC article. Review.
-
Immunopeptidomics reveals determinants of Mycobacterium tuberculosis antigen presentation on MHC class I.Elife. 2023 Apr 19;12:e84070. doi: 10.7554/eLife.84070. Elife. 2023. PMID: 37073954 Free PMC article.
-
Predicting susceptibility to tuberculosis based on gene expression profiling in dendritic cells.Sci Rep. 2017 Jul 18;7(1):5702. doi: 10.1038/s41598-017-05878-w. Sci Rep. 2017. PMID: 28720766 Free PMC article.
-
Antigen presentation by MHC-E: a putative target for vaccination?Trends Immunol. 2022 May;43(5):355-365. doi: 10.1016/j.it.2022.03.002. Epub 2022 Mar 31. Trends Immunol. 2022. PMID: 35370095 Free PMC article. Review.
-
High-frequency vaccine-induced CD8⁺ T cells specific for an epitope naturally processed during infection with Mycobacterium tuberculosis do not confer protection.Eur J Immunol. 2014 Jun;44(6):1699-709. doi: 10.1002/eji.201344358. Epub 2014 Mar 27. Eur J Immunol. 2014. PMID: 24677089 Free PMC article.
References
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93–129. - PubMed
-
- Grotzke JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:776–788. - PubMed
-
- Stegelmann F, Bastian M, Swoboda K, Bhat R, Kiessler V, Krensky AM, Roellinghoff M, Modlin RL, Stenger S. Coordinate expression of CC chemokine ligand 5, granulysin, and perforin in CD8+ T cells provides a host defense mechanism against Mycobacterium tuberculosis. J. Immunol. 2005;175:7474–7483. - PubMed
-
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. - PubMed
-
- Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000;164:2016–2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

