Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound
- PMID: 20740666
- PMCID: PMC2950102
- DOI: 10.1002/mrm.22411
Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound
Abstract
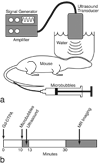
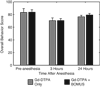
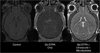
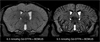
The use of contrast agents for neuroimaging is limited by the blood-brain barrier (BBB), which restricts entry into the brain. To administer imaging agents to the brain of rats, intracarotid infusions of hypertonic mannitol have been used to open the BBB. However, this technically challenging approach is invasive, opens only a limited region of the BBB, and is difficult to extend to mice. In this work, the BBB was opened in mice, using unfocused ultrasound combined with an injection of microbubbles. This technique has several notable features: it (a) can be performed transcranially in mice; (b) takes only 3 min and uses only commercially available components; (c) opens the BBB throughout the brain; (d) causes no observed histologic damage or changes in behavior (with peak-negative acoustic pressures of 0.36 MPa); and (e) allows recovery of the BBB within 4 h. Using this technique, Gadopentetate Dimeglumine (Gd-DTPA) was administered to the mouse brain parenchyma, thereby shortening T(1) and enabling the acquisition of high-resolution (52 × 52 × 100 micrometers(3)) images in 51 min in vivo. By enabling the administration of both existing anatomic contrast agents and the newer molecular/sensing contrast agents, this technique may be useful for the study of mouse models of neurologic function and pathology with MRI.
Figures






Similar articles
-
Blood-brain barrier (BBB) disruption using a diagnostic ultrasound scanner and Definity in Mice.Ultrasound Med Biol. 2009 Aug;35(8):1298-308. doi: 10.1016/j.ultrasmedbio.2009.03.012. Epub 2009 Jul 9. Ultrasound Med Biol. 2009. PMID: 19545939 Free PMC article.
-
A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI.Magn Reson Med. 2012 Mar;67(3):769-77. doi: 10.1002/mrm.23063. Epub 2011 Aug 19. Magn Reson Med. 2012. PMID: 21858862 Free PMC article.
-
Paramagnetic perfluorocarbon-filled albumin-(Gd-DTPA) microbubbles for the induction of focused-ultrasound-induced blood-brain barrier opening and concurrent MR and ultrasound imaging.Phys Med Biol. 2012 May 7;57(9):2787-802. doi: 10.1088/0031-9155/57/9/2787. Epub 2012 Apr 18. Phys Med Biol. 2012. PMID: 22510713
-
Contrast-enhanced ultrasound imaging for the detection of focused ultrasound-induced blood-brain barrier opening.Theranostics. 2014 Aug 1;4(10):1014-25. doi: 10.7150/thno.9575. eCollection 2014. Theranostics. 2014. PMID: 25161701 Free PMC article.
-
Fast in vivo imaging of amyloid plaques using μ-MRI Gd-staining combined with ultrasound-induced blood-brain barrier opening.Neuroimage. 2013 Oct 1;79:288-94. doi: 10.1016/j.neuroimage.2013.04.106. Epub 2013 May 7. Neuroimage. 2013. PMID: 23660031
Cited by
-
7.0-T magnetic resonance imaging characterization of acute blood-brain-barrier disruption achieved with intracranial irreversible electroporation.PLoS One. 2012;7(11):e50482. doi: 10.1371/journal.pone.0050482. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226293 Free PMC article.
-
Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system.Adv Drug Deliv Rev. 2014 Jun;72:94-109. doi: 10.1016/j.addr.2014.01.008. Epub 2014 Jan 22. Adv Drug Deliv Rev. 2014. PMID: 24462453 Free PMC article. Review.
-
Manganese enhanced MRI (MEMRI): neurophysiological applications.Rev Neurosci. 2011;22(6):675-94. doi: 10.1515/RNS.2011.048. Epub 2011 Nov 18. Rev Neurosci. 2011. PMID: 22098448 Free PMC article. Review.
-
Drug and gene delivery across the blood-brain barrier with focused ultrasound.J Control Release. 2015 Dec 10;219:61-75. doi: 10.1016/j.jconrel.2015.08.059. Epub 2015 Sep 8. J Control Release. 2015. PMID: 26362698 Free PMC article. Review.
-
Distribution and Diffusion of Macromolecule Delivery to the Brain via Focused Ultrasound using Magnetic Resonance and Multispectral Fluorescence Imaging.Ultrasound Med Biol. 2020 Jan;46(1):122-136. doi: 10.1016/j.ultrasmedbio.2019.08.024. Epub 2019 Oct 2. Ultrasound Med Biol. 2020. PMID: 31585767 Free PMC article.
References
-
- Johnson GA, Cofer GP, Gewalt SL, Hedlund LW. Morphologic phenotyping with MR microscopy: The visible mouse. Radiology. 2002;222(3):789–793. - PubMed
-
- Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, Neuwelt EA. Chemotherapy delivery issues in central nervous system malignancy: A reality check. Journal of Clinical Oncology. 2007;25(16):2295–2305. - PubMed
-
- Doolittle ND, Peereboom DM, Christoforidis GA, Hall WA, Palmieri D, Brock PR, Campbell K, Dickey DT, Muldoon LL, O'Neill BP, Peterson DR, Pollock B, Soussain C, Smith Q, Tyson RM, Neuwelt EA. Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the eleventh annual blood-brain barrier consortium meeting. Journal of Neuro-Oncology. 2007;81(1):81–91. - PubMed
-
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery. 1998;42(5):1083–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

