Nasal septum perforation: a side effect of bevacizumab chemotherapy in breast cancer patients
- PMID: 20736943
- PMCID: PMC2966623
- DOI: 10.1038/sj.bjc.6605828
Nasal septum perforation: a side effect of bevacizumab chemotherapy in breast cancer patients
Abstract
Background: Bevacizumab is an anti-vascular endothelial growth factor approved in association with paclitaxel or docetaxel as first line in patients (pts) with metastatic breast cancer. Rare cases of nasal septum perforations have been reported. We report our experience of nasal perforation in breast cancer pts receiving bevacizumab and chemotherapy either in the adjuvant or in the metastatic settings.
Methods: Between 1 January and 31 December 2009, 70 pts received bevacizumab together with chemotherapy. All the pts who had received bevacizumab were referred to the ENT specialist. Symptoms potentially related were looked for. Side effects were graded according to CTCAE.
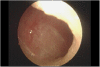
Results: Five nasal septum perforations were diagnosed (5 out of 70; 7.14%). Bevacizumab dose was 15 mg kg(-1) 3 weekly. Three pts were metastatic. Bevacizumab was associated with docetaxel (100 mg m(-2) every 3 weeks) in two pts and with weekly paclitaxel in one. The last two pts received bevacizumab in combination with anthracyclin and then taxanes in the adjuvant setting. In these two cases, nasal septum perforation occurred at the time of docetaxel treatment.
Conclusion: A high incidence of nasal septum perforation has been shown in pts with breast cancer receiving bevacizumab together with chemotherapy. Several mechanisms could be involved (mucositis, delayed tissue repair, antiangiogenic action of taxanes).
Figures
Similar articles
-
[Nasal septum perforation and bevacizumab].Rev Med Interne. 2011 Apr;32(4):e43-5. doi: 10.1016/j.revmed.2010.04.015. Epub 2010 Jun 17. Rev Med Interne. 2011. PMID: 21470582 French.
-
Nasal septum perforation in a bevacizumab-treated patient with metastatic breast cancer.Oncologist. 2006 Nov-Dec;11(10):1070-1. doi: 10.1634/theoncologist.11-10-1070. Oncologist. 2006. PMID: 17110625 No abstract available.
-
A patient presenting nasal septum perforation during bevacizumab-containing chemotherapy for advanced breast cancer.Breast Cancer. 2011 Jul;18(3):226-30. doi: 10.1007/s12282-011-0255-8. Epub 2011 Feb 11. Breast Cancer. 2011. PMID: 21312011 Review.
-
Nasal septum perforation and bevacizumab.Med Oncol. 2011 Mar;28(1):89-93. doi: 10.1007/s12032-010-9464-9. Epub 2010 Mar 6. Med Oncol. 2011. PMID: 20213219
-
Bevacizumab: a review of its use in combination with paclitaxel or capecitabine as first-line therapy for HER2-negative metastatic breast cancer.Drugs. 2011 Nov 12;71(16):2213-29. doi: 10.2165/11207720-000000000-00000. Drugs. 2011. PMID: 22035518 Review.
Cited by
-
Precision medicine based on tumorigenic signaling pathways for triple-negative breast cancer.Oncol Lett. 2018 Oct;16(4):4984-4996. doi: 10.3892/ol.2018.9290. Epub 2018 Aug 10. Oncol Lett. 2018. PMID: 30250564 Free PMC article. Review.
-
Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy.Int J Nanomedicine. 2019 Feb 25;14:1489-1501. doi: 10.2147/IJN.S195504. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30880960 Free PMC article.
-
pH-Responsive Cross-Linked Low Molecular Weight Polyethylenimine as an Efficient Gene Vector for Delivery of Plasmid DNA Encoding Anti-VEGF-shRNA for Tumor Treatment.Front Oncol. 2018 Sep 25;8:354. doi: 10.3389/fonc.2018.00354. eCollection 2018. Front Oncol. 2018. PMID: 30319959 Free PMC article.
-
FA2H Exhibits Tumor Suppressive Roles on Breast Cancers via Cancer Stemness Control.Front Oncol. 2019 Oct 24;9:1089. doi: 10.3389/fonc.2019.01089. eCollection 2019. Front Oncol. 2019. PMID: 31709178 Free PMC article.
-
Dysphonia induced by anti-angiogenic compounds.Invest New Drugs. 2014 Aug;32(4):774-82. doi: 10.1007/s10637-013-0049-2. Epub 2013 Dec 18. Invest New Drugs. 2014. PMID: 24343672 Review.
References
-
- Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2(11): 1843–1849 - PubMed
-
- Brufsky A, Bondarenko I, Smirnov V, Hurvitz S, Perez E, Ponomarova O, Vynnychenko I, Swamy R, Mu H, Rivera R (2009) RIBBON-2: A Randomized, Double-Blind, Placebo-Controlled, Phase III Trial Evaluating the Efficacy and Safety of Bevacizumab In Combination with Chemotherapy for Second-Line Treatment of HER2-Negative Metastatic Breast Cancer. Cancer Res 69(24 suppl): 495s. (abst 42) - PubMed
-
- Burkart CM, Grisel JJ, Hom DB (2008) Spontaneous nasal septal perforation with antiangiogenic bevacizumab therapy. Laryngoscope 118(9): 1539–1541 - PubMed
-
- Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS 31 March 2003 (http://ctep.cancer.gov), Published on 9 August 2006
-
- Fakih MG, Lombardo JC (2006) Bevacizumab-induced nasal septum perforation. Oncologist 11(1): 85–86 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous