Mitophagy and Parkinson's disease: the PINK1-parkin link
- PMID: 20736035
- PMCID: PMC3925795
- DOI: 10.1016/j.bbamcr.2010.08.007
Mitophagy and Parkinson's disease: the PINK1-parkin link
Abstract
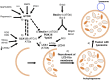
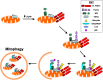
The study of rare, inherited mutations underlying familial forms of Parkinson's disease has provided insight into the molecular mechanisms of disease pathogenesis. Mutations in these genes have been functionally linked to several key molecular pathways implicated in other neurodegenerative disorders, including mitochondrial dysfunction, protein accumulation and the autophagic-lysosomal pathway. In particular, the mitochondrial kinase PINK1 and the cytosolic E3 ubiquitin ligase parkin act in a common pathway to regulate mitochondrial function. In this review we discuss the recent evidence suggesting that the PINK1/parkin pathway also plays a critical role in the autophagic removal of damaged mitochondria-mitophagy. This article is part of a Special Issue entitled Mitochondria: the deadly organelle.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Regulation by mitophagy.Int J Biochem Cell Biol. 2014 Aug;53:147-50. doi: 10.1016/j.biocel.2014.05.012. Epub 2014 May 16. Int J Biochem Cell Biol. 2014. PMID: 24842103 Review.
-
The role of PINK1-Parkin in mitochondrial quality control.Nat Cell Biol. 2024 Oct;26(10):1639-1651. doi: 10.1038/s41556-024-01513-9. Epub 2024 Oct 2. Nat Cell Biol. 2024. PMID: 39358449 Review.
-
Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease.Curr Opin Neurobiol. 2007 Jun;17(3):331-7. doi: 10.1016/j.conb.2007.04.010. Epub 2007 May 11. Curr Opin Neurobiol. 2007. PMID: 17499497 Review.
-
PINK1 import regulation at a crossroad of mitochondrial fate: the molecular mechanisms of PINK1 import.J Biochem. 2020 Mar 1;167(3):217-224. doi: 10.1093/jb/mvz069. J Biochem. 2020. PMID: 31504668 Review.
Cited by
-
Microtubule affinity-regulating kinase 2 (MARK2) turns on phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) at Thr-313, a mutation site in Parkinson disease: effects on mitochondrial transport.J Biol Chem. 2012 Mar 9;287(11):8174-86. doi: 10.1074/jbc.M111.262287. Epub 2012 Jan 11. J Biol Chem. 2012. PMID: 22238344 Free PMC article.
-
Increase of autophagy and attenuation of apoptosis by Salvigenin promote survival of SH-SY5Y cells following treatment with H₂O₂.Mol Cell Biochem. 2012 Dec;371(1-2):9-22. doi: 10.1007/s11010-012-1416-6. Epub 2012 Aug 17. Mol Cell Biochem. 2012. PMID: 22899171
-
Dysfunction of the autophagy/lysosomal degradation pathway is a shared feature of the genetic synucleinopathies.FASEB J. 2013 Sep;27(9):3424-9. doi: 10.1096/fj.12-223842. Epub 2013 May 16. FASEB J. 2013. PMID: 23682122 Free PMC article. Review.
-
Mapping the research of mitochondria and Parkinson's disease: a bibliometric analysis.Front Neurol. 2024 Sep 16;15:1413762. doi: 10.3389/fneur.2024.1413762. eCollection 2024. Front Neurol. 2024. PMID: 39350973 Free PMC article.
-
ALS Patient Stem Cells for Unveiling Disease Signatures of Motoneuron Susceptibility: Perspectives on the Deadly Mitochondria, ER Stress and Calcium Triad.Front Cell Neurosci. 2015 Nov 19;9:448. doi: 10.3389/fncel.2015.00448. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26635528 Free PMC article. Review.
References
-
- Lesage S., Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–59. - PubMed
-
- Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Schapira A.H. Mitochondrial complex I deficiency in Parkinson's disease. Adv. Neurol. 1993;60:288–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

