Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice
- PMID: 20727895
- PMCID: PMC3031737
- DOI: 10.1053/j.gastro.2010.08.020
Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice
Abstract
Background & aims: Liver inflammation and injury are mediated by the innate immune response, which is regulated by Toll-like receptors (TLR). Activation of TLR9 induces type I interferons (IFNs) via the interferon regulatory factor (IRF)-7. We investigated the roles of type I IFNs in TLR9-associated liver injury.
Methods: Wild-type (WT), IRF7-deficient, and IFN-α/β receptor 1 (IFNAR1)-deficient mice were stimulated with TLR9 or TLR2 ligands. Findings from mice were verified in cultured hepatocytes and liver mononuclear cells (LMNCs) as well as in vivo experiments using recombinant type I IFN and interleukin-1 receptor antagonist (IL-1ra).
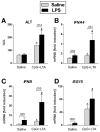
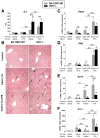
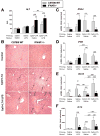
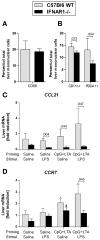
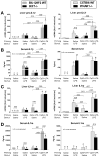
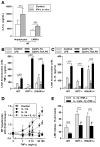
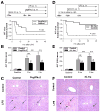
Results: Type I IFNs were up-regulated during TLR9-associated liver injury in WT mice. IRF7- and IFNAR1-deficient mice, which have disruptions in type I IFN production or signaling, respectively, had increased liver damage and inflammation, decreased recruitment of dendritic cells, and increased production of tumor necrosis factor α by LMNCs. These findings indicate that type I IFNs have anti-inflammatory activities in liver. IL-1ra, which is produced by LMNCs and hepatocytes, is an IFN-regulated antagonist of the proinflammatory cytokine IL-1β; IRF7- and IFNAR1-deficient mice had decreased levels of IL-1ra compared with WT mice. IL-1ra protected cultured hepatocytes from IL-1β-mediated sensitization to cytotoxicity from tumor necrosis factor α. In vivo exposure to type I IFN, which induced IL-1ra, or administration of IL-1ra reduced TLR9-associated liver injury; the protective effect of type I IFNs therefore appears to be mediated by IFN-dependent induction of IL-1ra.
Conclusions: Type I IFNs have anti-inflammatory effects mediated by endogenous IL-1ra, which regulates the extent of TLR9-induced liver damage. Type I IFN signaling is therefore required for protection from immune-mediated liver injury.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis.Hepatology. 2014 Jul;60(1):237-49. doi: 10.1002/hep.26981. Epub 2014 May 27. Hepatology. 2014. PMID: 24375615 Free PMC article.
-
Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNF)-alpha in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice.Immunol Lett. 2009 Apr 27;123(2):138-43. doi: 10.1016/j.imlet.2009.03.004. Epub 2009 Mar 21. Immunol Lett. 2009. PMID: 19428561
-
Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells.Hepatology. 2011 Feb;53(2):649-60. doi: 10.1002/hep.24059. Epub 2011 Jan 10. Hepatology. 2011. PMID: 21274885 Free PMC article.
-
Endogenous, or therapeutically induced, type I interferon responses differentially modulate Th1/Th17-mediated autoimmunity in the CNS.Immunol Cell Biol. 2012 May;90(5):505-9. doi: 10.1038/icb.2012.8. Epub 2012 Mar 20. Immunol Cell Biol. 2012. PMID: 22430251 Free PMC article. Review.
-
99mTc-Type II tumor necrosis receptor-Fc-interlukin-1 receptor antagonist fusion protein.2013 May 1 [updated 2013 May 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2013 May 1 [updated 2013 May 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23720862 Free Books & Documents. Review.
Cited by
-
A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet.Sci Rep. 2016 Apr 14;6:24399. doi: 10.1038/srep24399. Sci Rep. 2016. PMID: 27075683 Free PMC article.
-
Kuhuang alleviates liver fibrosis by modulating gut microbiota-mediated hepatic IFN signaling and bile acid synthesis.Front Pharmacol. 2022 Dec 13;13:1080226. doi: 10.3389/fphar.2022.1080226. eCollection 2022. Front Pharmacol. 2022. PMID: 36582518 Free PMC article.
-
Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role.Biomedicines. 2022 Feb 25;10(3):550. doi: 10.3390/biomedicines10030550. Biomedicines. 2022. PMID: 35327352 Free PMC article. Review.
-
Tamarix chinensis Lour inhibits chronic ethanol-induced liver injury in mice.World J Gastroenterol. 2020 Mar 28;26(12):1286-1297. doi: 10.3748/wjg.v26.i12.1286. World J Gastroenterol. 2020. PMID: 32256017 Free PMC article.
-
Why and How Is Hyperferritinemic Sepsis Different From Sepsis Without Hyperferritinemia?Pediatr Crit Care Med. 2020 May;21(5):509-512. doi: 10.1097/PCC.0000000000002285. Pediatr Crit Care Med. 2020. PMID: 32358338 Free PMC article. No abstract available.
References
-
- Minino AM, Heron MP, Murphy SL, et al. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. - PubMed
-
- Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–97. - PubMed
-
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–35. - PubMed
-
- Huck S, Deveaud E, Namane A, et al. Abnormal DNA methylation and deoxycytosine-deoxyguanine content in nucleosomes from lymphocytes undergoing apoptosis. Faseb J. 1999;13:1415–22. - PubMed
-
- Gustot T, Lemmers A, Moreno C, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

