Cellular strategies for regulating DNA supercoiling: a single-molecule perspective
- PMID: 20723754
- PMCID: PMC2997354
- DOI: 10.1016/j.cell.2010.08.001
Cellular strategies for regulating DNA supercoiling: a single-molecule perspective
Abstract
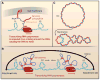
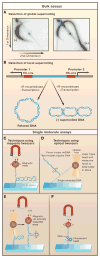
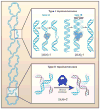
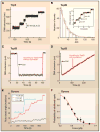
Entangling and twisting of cellular DNA (i.e., supercoiling) are problems inherent to the helical structure of double-stranded DNA. Supercoiling affects transcription, DNA replication, and chromosomal segregation. Consequently the cell must fine-tune supercoiling to optimize these key processes. Here, we summarize how supercoiling is generated and review experimental and theoretical insights into supercoil relaxation. We distinguish between the passive dissipation of supercoils by diffusion and the active removal of supercoils by topoisomerase enzymes. We also review single-molecule studies that elucidate the timescales and mechanisms of supercoil removal.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Control of bacterial DNA supercoiling.Mol Microbiol. 1992 Feb;6(4):425-33. doi: 10.1111/j.1365-2958.1992.tb01486.x. Mol Microbiol. 1992. PMID: 1313943 Review.
-
[DNA supercoiling and topoisomerases in Escherichia coli].Rev Latinoam Microbiol. 1995 Jul-Sep;37(3):291-304. Rev Latinoam Microbiol. 1995. PMID: 8850348 Review. Spanish.
-
A multiscale dynamic model of DNA supercoil relaxation by topoisomerase IB.Biophys J. 2011 Apr 20;100(8):2016-23. doi: 10.1016/j.bpj.2011.03.003. Biophys J. 2011. PMID: 21504738 Free PMC article.
-
Rotation of DNA around intact strand in human topoisomerase I implies distinct mechanisms for positive and negative supercoil relaxation.Nucleic Acids Res. 2005 Nov 27;33(20):6621-34. doi: 10.1093/nar/gki935. Print 2005. Nucleic Acids Res. 2005. PMID: 16314322 Free PMC article.
-
DNA supercoiling during ATP-dependent DNA translocation by the type I restriction enzyme EcoAI.J Mol Biol. 2000 Jan 28;295(4):1089-99. doi: 10.1006/jmbi.1999.3414. J Mol Biol. 2000. PMID: 10656812
Cited by
-
DNA Manipulation and Single-Molecule Imaging.Molecules. 2021 Feb 17;26(4):1050. doi: 10.3390/molecules26041050. Molecules. 2021. PMID: 33671359 Free PMC article. Review.
-
High-Resolution Genome-Wide Maps Reveal Widespread Presence of Torsional Insulation.bioRxiv [Preprint]. 2025 Jan 4:2024.10.11.617876. doi: 10.1101/2024.10.11.617876. bioRxiv. 2025. PMID: 39416127 Free PMC article. Preprint.
-
Psoralen mapping reveals a bacterial genome supercoiling landscape dominated by transcription.Nucleic Acids Res. 2022 May 6;50(8):4436-4449. doi: 10.1093/nar/gkac244. Nucleic Acids Res. 2022. PMID: 35420137 Free PMC article.
-
Application of Plasmid Engineering to Enhance Yield and Quality of Plasmid for Vaccine and Gene Therapy.Bioengineering (Basel). 2019 Jun 19;6(2):54. doi: 10.3390/bioengineering6020054. Bioengineering (Basel). 2019. PMID: 31248216 Free PMC article.
-
The dynamic interplay between DNA topoisomerases and DNA topology.Biophys Rev. 2016 Nov;8(Suppl 1):101-111. doi: 10.1007/s12551-016-0240-8. Epub 2016 Nov 14. Biophys Rev. 2016. PMID: 28510219 Free PMC article. Review.
References
-
- Bishop AI, Nieminen TA, Heckenberg NR, Rubinsztein-Dunlop H. Optical application and measurement of torque on microparticles of isotropic nonabsorbing material. Phys Rev A. 2003;68:033802.
-
- Bohbot-Raviv Y, Zhao WZ, Feingold M, Wiggins CH, Granek R. Relaxation dynamics of semiflexible polymers. Phys Rev Lett. 2004;92:098101. - PubMed
-
- Brown PO, Cozzarelli NR. Sign Inversion Mechanism for Enzymatic Supercoiling of DNA. Science. 1979;206:1081–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

