Cytoskeletal dysfunction dominates in DAP12-deficient osteoclasts
- PMID: 20720152
- PMCID: PMC2923570
- DOI: 10.1242/jcs.069872
Cytoskeletal dysfunction dominates in DAP12-deficient osteoclasts
Abstract
Despite evidence that DAP12 regulates osteoclasts, mice lacking the ITAM-bearing protein exhibit only mild osteopetrosis. Alternatively, Dap12(-/-) mice, also lacking FcRgamma, are severely osteopetrotic, suggesting that FcRgamma compensates for DAP12 deficiency in the bone-resorbing polykaryons. Controversy exists, however, as to whether these co-stimulatory molecules regulate differentiation of osteoclasts or the capacity of the mature cell to degrade bone. We find that Dap12(-/-) osteoclasts differentiate normally when generated on osteoblasts but have a dysfunctional cytoskeleton, impairing their ability to transmigrate through the osteoblast layer and resorb bone. To determine whether the FcRgamma co-receptor, OSCAR mediates osteoclast function in the absence of DAP12, we overexpressed OSCAR fused to FLAG (OSCAR-FLAG), in Dap12(-/-) osteoclasts. OSCAR-FLAG partially rescues the abnormal cytoskeleton of Dap12(-/-) osteoclasts grown on bone, but not those grown on osteoblasts. Thus, cytoskeletal dysfunction, and not arrested differentiation, is the dominant consequence of DAP12 deficiency in osteoclasts. The failure of osteoblasts to normalize Dap12(-/-) osteoclasts indicates that functionally relevant quantities of OSCAR ligand do not reside in bone-forming cells.
Figures

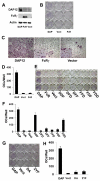
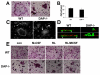

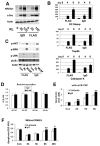
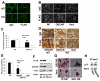
Similar articles
-
Absence of Dap12 and the αvβ3 integrin causes severe osteopetrosis.J Cell Biol. 2015 Jan 5;208(1):125-36. doi: 10.1083/jcb.201410123. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547154 Free PMC article.
-
The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6158-63. doi: 10.1073/pnas.0401602101. Epub 2004 Apr 8. Proc Natl Acad Sci U S A. 2004. PMID: 15073337 Free PMC article.
-
Lymphocytes and the Dap12 adaptor are key regulators of osteoclast activation associated with gonadal failure.PLoS One. 2007 Jul 4;2(7):e585. doi: 10.1371/journal.pone.0000585. PLoS One. 2007. PMID: 17611620 Free PMC article.
-
[Regulation of osteoclast development by immunoglobulin-like receptors].Nihon Rinsho. 2005 Sep;63(9):1562-8. Nihon Rinsho. 2005. PMID: 16164212 Review. Japanese.
-
A Comprehensive Review of Immunoreceptor Regulation of Osteoclasts.Clin Rev Allergy Immunol. 2016 Aug;51(1):48-58. doi: 10.1007/s12016-015-8521-8. Clin Rev Allergy Immunol. 2016. PMID: 26573914 Free PMC article. Review.
Cited by
-
NFAM1 signaling enhances osteoclast formation and bone resorption activity in Paget's disease of bone.Bone. 2017 Aug;101:236-244. doi: 10.1016/j.bone.2017.05.013. Epub 2017 May 12. Bone. 2017. PMID: 28506889 Free PMC article.
-
Absence of Dap12 and the αvβ3 integrin causes severe osteopetrosis.J Cell Biol. 2015 Jan 5;208(1):125-36. doi: 10.1083/jcb.201410123. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547154 Free PMC article.
-
Measles virus nucleocapsid protein modulates the Signal Regulatory Protein-β1 (SIRPβ1) to enhance osteoclast differentiation in Paget's disease of bone.Bone Rep. 2016 Jun 14;7:26-32. doi: 10.1016/j.bonr.2016.06.002. eCollection 2017 Dec. Bone Rep. 2016. PMID: 28840181 Free PMC article.
-
Talin1 and Rap1 are critical for osteoclast function.Mol Cell Biol. 2013 Feb;33(4):830-44. doi: 10.1128/MCB.00790-12. Epub 2012 Dec 10. Mol Cell Biol. 2013. PMID: 23230271 Free PMC article.
-
Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12).J Biol Chem. 2012 May 18;287(21):17493-17502. doi: 10.1074/jbc.M111.324194. Epub 2012 Mar 26. J Biol Chem. 2012. PMID: 22451653 Free PMC article.
References
-
- Baron R. (2004). Arming the osteoclast. Nat. Med. 10, 458-460 - PubMed
-
- Colonna M., Turnbull I., Klesney-Tait J. (2007). The enigmatic function of TREM-2 in osteoclastogenesis. Adv. Exp. Med. Biol. 602, 97-105 - PubMed
-
- Faccio R., Zou W., Colaianni G., Teitelbaum S. L., Ross F. P. (2003). High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J. Cell. Biochem. 90, 871-883 - PubMed
-
- Faccio R., Teitelbaum S. L., Fujikawa K., Chappel J., Zallone A., Tybulewicz V. L., Ross F. P., Swat W. (2005). Vav3 regulates osteoclast function and bone mass. Nat. Med. 11, 284-290 - PubMed
-
- Humphrey M. B., Lanier L. L., Nakamura M. C. (2005). Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Rev. 208, 50-65 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR032788/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- AR046523/AR/NIAMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- AR054618/AR/NIAMS NIH HHS/United States
- P30 DK056341-10/DK/NIDDK NIH HHS/United States
- AR057037/AR/NIAMS NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- HL084922/HL/NHLBI NIH HHS/United States
- R01 AR057037/AR/NIAMS NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- R01 AR054618/AR/NIAMS NIH HHS/United States
- P50 HL084922/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

