Glutamate receptor ion channels: structure, regulation, and function
- PMID: 20716669
- PMCID: PMC2964903
- DOI: 10.1124/pr.109.002451
Glutamate receptor ion channels: structure, regulation, and function
Erratum in
- Pharmacol Rev. 2014 Oct;66(4):1141
Abstract
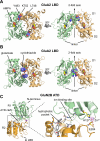
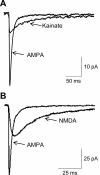
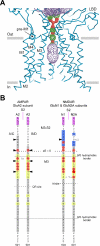
The mammalian ionotropic glutamate receptor family encodes 18 gene products that coassemble to form ligand-gated ion channels containing an agonist recognition site, a transmembrane ion permeation pathway, and gating elements that couple agonist-induced conformational changes to the opening or closing of the permeation pore. Glutamate receptors mediate fast excitatory synaptic transmission in the central nervous system and are localized on neuronal and non-neuronal cells. These receptors regulate a broad spectrum of processes in the brain, spinal cord, retina, and peripheral nervous system. Glutamate receptors are postulated to play important roles in numerous neurological diseases and have attracted intense scrutiny. The description of glutamate receptor structure, including its transmembrane elements, reveals a complex assembly of multiple semiautonomous extracellular domains linked to a pore-forming element with striking resemblance to an inverted potassium channel. In this review we discuss International Union of Basic and Clinical Pharmacology glutamate receptor nomenclature, structure, assembly, accessory subunits, interacting proteins, gene expression and translation, post-translational modifications, agonist and antagonist pharmacology, allosteric modulation, mechanisms of gating and permeation, roles in normal physiological function, as well as the potential therapeutic use of pharmacological agents acting at glutamate receptors.
Figures


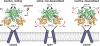








Similar articles
-
Functional analysis of Caenorhabditis elegans glutamate receptor subunits by domain transplantation.J Biol Chem. 2003 Nov 7;278(45):44691-701. doi: 10.1074/jbc.M305497200. Epub 2003 Aug 20. J Biol Chem. 2003. PMID: 12930835
-
Structure and gating of the glutamate receptor ion channel.Trends Neurosci. 2004 Jun;27(6):321-8. doi: 10.1016/j.tins.2004.04.005. Trends Neurosci. 2004. PMID: 15165736 Review.
-
Regulation of ligand-gated ion channels by protein phosphorylation.Adv Second Messenger Phosphoprotein Res. 1999;33:49-78. doi: 10.1016/s1040-7952(99)80005-6. Adv Second Messenger Phosphoprotein Res. 1999. PMID: 10218114 Review.
-
Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation.J Mol Biol. 2008 Oct 31;383(1):36-48. doi: 10.1016/j.jmb.2008.06.076. Epub 2008 Jul 3. J Mol Biol. 2008. PMID: 18625242
-
The Challenge of Interpreting Glutamate-Receptor Ion-Channel Structures.Biophys J. 2017 Nov 21;113(10):2143-2151. doi: 10.1016/j.bpj.2017.07.028. Epub 2017 Aug 24. Biophys J. 2017. PMID: 28844473 Free PMC article. Review.
Cited by
-
Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart-Brain Axis.Biomedicines. 2024 Oct 18;12(10):2387. doi: 10.3390/biomedicines12102387. Biomedicines. 2024. PMID: 39457698 Free PMC article. Review.
-
Kainate receptor post-translational modifications differentially regulate association with 4.1N to control activity-dependent receptor endocytosis.J Biol Chem. 2013 Mar 29;288(13):8952-65. doi: 10.1074/jbc.M112.440719. Epub 2013 Feb 11. J Biol Chem. 2013. PMID: 23400781 Free PMC article.
-
Redefining the classification of AMPA-selective ionotropic glutamate receptors.J Physiol. 2012 Jan 1;590(1):49-61. doi: 10.1113/jphysiol.2011.221689. Epub 2011 Nov 21. J Physiol. 2012. PMID: 22106175 Free PMC article. Review.
-
Structural prediction of GluN3 NMDA receptors.Front Physiol. 2024 Aug 20;15:1446459. doi: 10.3389/fphys.2024.1446459. eCollection 2024. Front Physiol. 2024. PMID: 39229618 Free PMC article.
-
Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity.J Biol Chem. 2013 Aug 16;288(33):24151-9. doi: 10.1074/jbc.M113.482000. Epub 2013 Jul 9. J Biol Chem. 2013. PMID: 23839940 Free PMC article.
References
-
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145 - PubMed
-
- Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E. (2009) Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8:613–618 - PubMed
-
- Abbott LF, Regehr WG. (2004) Synaptic computation. Nature 431:796–803 - PubMed
-
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, et al. (2005) Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci 22:1445–1454 - PubMed
-
- Abele R, Keinanen K, Madden DR. (2000) Agonist-induced isomerization in a glutamate receptor ligand-binding domain. A kinetic and mutagenetic analysis. J Biol Chem 275:21355–21363 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32-DA01504006/DA/NIDA NIH HHS/United States
- R37 NS036654/NS/NINDS NIH HHS/United States
- NS068464/NS/NINDS NIH HHS/United States
- R01 NS036654/NS/NINDS NIH HHS/United States
- R01 NS068464/NS/NINDS NIH HHS/United States
- NS036604/NS/NINDS NIH HHS/United States
- R01 NS065371/NS/NINDS NIH HHS/United States
- R01 EY016979-07/EY/NEI NIH HHS/United States
- R01 MH066892-07/MH/NIMH NIH HHS/United States
- R01 MH066892/MH/NIMH NIH HHS/United States
- NS036654/NS/NINDS NIH HHS/United States
- MH066892/MH/NIMH NIH HHS/United States
- T32 GM008602/GM/NIGMS NIH HHS/United States
- T32-GM008602/GM/NIGMS NIH HHS/United States
- EY01697905/EY/NEI NIH HHS/United States
- T32 ES012870/ES/NIEHS NIH HHS/United States
- R01 NS036604/NS/NINDS NIH HHS/United States
- T32-ES012870/ES/NIEHS NIH HHS/United States
- R01 EY016979/EY/NEI NIH HHS/United States
- NS065371/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
