Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer
- PMID: 20713879
- PMCID: PMC3296668
- DOI: 10.1200/JCO.2010.30.4154
Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer
Abstract
Purpose: To evaluate the safety and efficacy of IMC-A12, a human monoclonal antibody (mAb) that blocks insulin-like growth factor receptor-1 (IGF-1R), as monotherapy or in combination with cetuximab in patients with metastatic refractory anti-epidermal growth factor receptor (EGFR) mAb colorectal cancer.
Methods: A randomized, phase II study was performed in which patients in arm A received IMC-A12 10 mg/kg intravenously (IV) every 2 weeks, while patients in arm B received this same dose of IMC-A12 plus cetuximab 500 mg/m(2) IV every 2 weeks. Subsequently, arm C (same combination treatment as arm B) was added to include patients who had disease control on a prior anti-EGFR mAb and wild-type KRAS tumors. Archived pretreatment tumor tissue was obtained when possible for KRAS, PIK3CA, and BRAF genotyping, and immunohistochemistry was obtained for pAKT as well as IGF-1R.
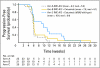
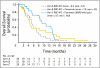
Results: Overall, 64 patients were treated (median age, 61 years; range, 40 to 84 years): 23 patients in arm A, 21 in arm B, and 20 in arm C. No antitumor activity was seen in the 23 patients treated with IMC-A12 monotherapy. Of the 21 patients randomly assigned to IMC-A12 plus cetuximab, one patient (with KRAS wild type) achieved a partial response, with disease control lasting 6.5 months. Arm C (all patients with KRAS wild type), however, showed no additional antitumor activity. Serious adverse events thought possibly related to IMC-A12 included a grade 2 infusion-related reaction (2%; one of 64 patients), thrombocytopenia (2%; one of 64 patients), grade 3 hyperglycemia (2%; one of 64 patients), and grade 1 pyrexia (2%, one of 64 patients).
Conclusion: IMC-A12 alone or in combination with cetuximab was insufficient to warrant additional study in patients with colorectal cancer refractory to EGFR inhibitors.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer.Int J Clin Oncol. 2013 Aug;18(4):670-7. doi: 10.1007/s10147-012-0422-8. Epub 2012 May 26. Int J Clin Oncol. 2013. PMID: 22638623
-
Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?Genet Med. 2013 Jul;15(7):517-27. doi: 10.1038/gim.2012.184. Epub 2013 Feb 21. Genet Med. 2013. PMID: 23429431
-
Co-expression of matrix metalloproteinase-7 (MMP-7) and phosphorylated insulin growth factor receptor I (pIGF-1R) correlates with poor prognosis in patients with wild-type KRAS treated with cetuximab or panitumumab: a GEMCAD study.Cancer Biol Ther. 2011 Jan 15;11(2):177-83. doi: 10.4161/cbt.11.2.13839. Epub 2011 Jan 15. Cancer Biol Ther. 2011. PMID: 21099348
-
Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab.Oncologist. 2012;17(1):14. doi: 10.1634/theoncologist.2011-0452. Epub 2011 Dec 30. Oncologist. 2012. PMID: 22210091 Free PMC article. Clinical Trial.
-
Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer.J Natl Cancer Inst. 2009 Oct 7;101(19):1308-24. doi: 10.1093/jnci/djp280. Epub 2009 Sep 8. J Natl Cancer Inst. 2009. PMID: 19738166 Free PMC article. Review.
Cited by
-
Insulin-like growth factor: current concepts and new developments in cancer therapy.Recent Pat Anticancer Drug Discov. 2012 Jan;7(1):14-30. doi: 10.2174/157489212798357930. Recent Pat Anticancer Drug Discov. 2012. PMID: 21875414 Free PMC article. Review.
-
Novel C6-substituted 1,3,4-oxadiazinones as potential anti-cancer agents.Oncotarget. 2015 Dec 1;6(38):40598-610. doi: 10.18632/oncotarget.5839. Oncotarget. 2015. PMID: 26515601 Free PMC article.
-
Combating resistance to anti-IGFR antibody by targeting the integrin β3-Src pathway.J Natl Cancer Inst. 2013 Oct 16;105(20):1558-70. doi: 10.1093/jnci/djt263. Epub 2013 Oct 3. J Natl Cancer Inst. 2013. PMID: 24092920 Free PMC article.
-
The association of TP53 mutations with the resistance of colorectal carcinoma to the insulin-like growth factor-1 receptor inhibitor picropodophyllin.BMC Cancer. 2013 Nov 4;13:521. doi: 10.1186/1471-2407-13-521. BMC Cancer. 2013. PMID: 24182354 Free PMC article.
-
Downregulation of IGFBP2 is associated with resistance to IGF1R therapy in rhabdomyosarcoma.Oncogene. 2014 Dec 11;33(50):5697-705. doi: 10.1038/onc.2013.509. Epub 2013 Dec 2. Oncogene. 2014. PMID: 24292683 Free PMC article.
References
-
- Wang Y, Sun Y. Insulin-like growth factor receptor-1 as an anti-cancer target: Blocking transformation and inducing apoptosis. Curr Cancer Drug Targets. 2002;2:191–207. - PubMed
-
- Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: From basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. - PubMed
-
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. - PubMed
-
- Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous