Reduction in structural disorder and functional complexity in the thermal adaptation of prokaryotes
- PMID: 20711457
- PMCID: PMC2920320
- DOI: 10.1371/journal.pone.0012069
Reduction in structural disorder and functional complexity in the thermal adaptation of prokaryotes
Abstract
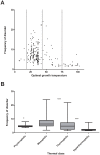
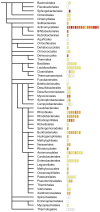
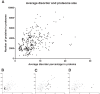
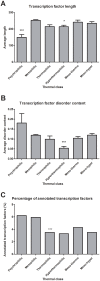
Genomic correlates of evolutionary adaptation to very low or very high optimal growth temperature (OGT) values have been the subject of many studies. Whereas these provided a protein-structural rationale of the activity and stability of globular proteins/enzymes, the point has been neglected that adaptation to extreme temperatures could also have resulted from an increased use of intrinsically disordered proteins (IDPs), which are resistant to these conditions in vitro. Contrary to these expectations, we found a conspicuously low level of structural disorder in bacteria of very high (and very low) OGT values. This paucity of disorder does not reflect phylogenetic relatedness, i.e. it is a result of genuine adaptation to extreme conditions. Because intrinsic disorder correlates with important regulatory functions, we asked how these bacteria could exist without IDPs by studying transcription factors, known to harbor a lot of function-related intrinsic disorder. Hyperthermophiles have much less transcription factors, which have reduced disorder compared to their mesophilic counterparts. On the other hand, we found by systematic categorization of proteins with long disordered regions that there are certain functions, such as translation and ribosome biogenesis that depend on structural disorder even in hyperthermophiles. In all, our observations suggest that adaptation to extreme conditions is achieved by a significant functional simplification, apparent at both the level of the genome and individual genes/proteins.
Conflict of interest statement
Figures
Similar articles
-
Some like it cold: biocatalysis at low temperatures.FEMS Microbiol Rev. 2004 Feb;28(1):25-42. doi: 10.1016/j.femsre.2003.07.003. FEMS Microbiol Rev. 2004. PMID: 14975528 Review.
-
Analysis of tRNA composition and folding in psychrophilic, mesophilic and thermophilic genomes: indications for thermal adaptation.FEMS Microbiol Lett. 2010 Apr;305(2):100-8. doi: 10.1111/j.1574-6968.2010.01922.x. Epub 2010 Feb 5. FEMS Microbiol Lett. 2010. PMID: 20659165
-
Prevalent structural disorder carries signature of prokaryotic adaptation to oxic atmosphere.Gene. 2014 Sep 10;548(1):134-41. doi: 10.1016/j.gene.2014.07.002. Epub 2014 Jul 5. Gene. 2014. PMID: 24999584
-
Adaptation of proteins from hyperthermophiles to high pressure and high temperature.J Mol Microbiol Biotechnol. 1999 Aug;1(1):101-5. J Mol Microbiol Biotechnol. 1999. PMID: 10941791 Review.
-
Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water.Adv Biochem Eng Biotechnol. 1998;61:37-85. doi: 10.1007/BFb0102289. Adv Biochem Eng Biotechnol. 1998. PMID: 9670797 Review.
Cited by
-
Mesophiles vs. Thermophiles: Untangling the Hot Mess of Intrinsically Disordered Proteins and Growth Temperature of Bacteria.Int J Mol Sci. 2024 Feb 7;25(4):2000. doi: 10.3390/ijms25042000. Int J Mol Sci. 2024. PMID: 38396678 Free PMC article.
-
Growth temperature and genome size in bacteria are negatively correlated, suggesting genomic streamlining during thermal adaptation.Genome Biol Evol. 2013;5(5):966-77. doi: 10.1093/gbe/evt050. Genome Biol Evol. 2013. PMID: 23563968 Free PMC article.
-
Arabidopsis Heat Stress-Induced Proteins Are Enriched in Electrostatically Charged Amino Acids and Intrinsically Disordered Regions.Int J Mol Sci. 2018 Aug 3;19(8):2276. doi: 10.3390/ijms19082276. Int J Mol Sci. 2018. PMID: 30081447 Free PMC article.
-
Common protein sequence signatures associate with Sclerotinia borealis lifestyle and secretion in fungal pathogens of the Sclerotiniaceae.Front Plant Sci. 2015 Sep 24;6:776. doi: 10.3389/fpls.2015.00776. eCollection 2015. Front Plant Sci. 2015. PMID: 26442085 Free PMC article.
-
Excavating the functionally crucial active-site residues of the DXS protein of Bacillus subtilis by exploring its closest homologues.J Genet Eng Biotechnol. 2020 Nov 26;18(1):76. doi: 10.1186/s43141-020-00087-x. J Genet Eng Biotechnol. 2020. PMID: 33242110 Free PMC article.
References
-
- Deming JW. Psychrophiles and polar regions. Curr Opin Microbiol. 2002;5:301–309. - PubMed
-
- Blochl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch HW, et al. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 degrees C. Extremophiles. 1997;1:14–21. - PubMed
-
- Puigbo P, Pasamontes A, Garcia-Vallve S. Gaining and losing the thermophilic adaptation in prokaryotes. Trends Genet. 2008;24:10–14. - PubMed
-
- Szilagyi A, Zavodszky P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure. 2000;8:493–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

