Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus
- PMID: 20711354
- PMCID: PMC2920837
- DOI: 10.1371/journal.pone.0012137
Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus
Abstract
Background: Mosquito-borne diseases are a worldwide public health threat. Mosquitoes transmit viruses or parasites during feeding, along with salivary proteins that modulate host responses to facilitate both blood feeding and pathogen transmission. Understanding these earliest events in mosquito transmission of arboviruses by mosquitoes is essential for development and assessment of rational vaccine and treatment strategies. In this report, we compared host immune responses to chikungunya virus (CHIKV) transmission by (1) mosquito bite, or (2) by needle inoculation.
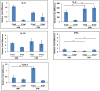
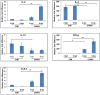
Methods and findings: Differential cytokine expression was measured using quantitative real-time RT-PCR, at sites of uninfected mosquito bites, CHIKV-infected mosquito bites, and needle-inoculated CHIKV. Both uninfected and CHIKV infected mosquitoes polarized host cytokine response to a TH2 profile. Compared to uninfected mosquito bites, expression of IL-4 induced by CHIKV-infected mosquitoes were 150 fold and 527.1 fold higher at 3 hours post feeding (hpf) and 6 hpf, respectively. A significant suppression of TH1 cytokines and TLR-3 was also observed. These significant differences may result from variation in the composition of uninfected and CHIKV-infected mosquito saliva. Needle injected CHIKV induced a robust interferon-gamma, no detectable IL-4, and a significant up-regulation of TLR-3.
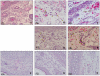
Conclusions: This report describes the first analysis of cutaneous cytokines in mice bitten by CHIKV-infected mosquitoes. Our data demonstrate contrasting immune activation in the response to CHIKV infection by mosquito bite or needle inoculation. The significant role of mosquito saliva in these earliest events of CHIKV transmission and infection are highlighted.
Conflict of interest statement
Figures
Similar articles
-
Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression.Infect Genet Evol. 2016 Jun;40:126-135. doi: 10.1016/j.meegid.2016.02.033. Epub 2016 Feb 27. Infect Genet Evol. 2016. PMID: 26925703
-
Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti.PLoS Negl Trop Dis. 2017 Jun 1;11(6):e0005654. doi: 10.1371/journal.pntd.0005654. eCollection 2017 Jun. PLoS Negl Trop Dis. 2017. PMID: 28570693 Free PMC article.
-
Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes.Parasit Vectors. 2016 Apr 23;9:227. doi: 10.1186/s13071-016-1505-6. Parasit Vectors. 2016. PMID: 27108077 Free PMC article.
-
Immune Response to Chikungunya Virus: Sex as a Biological Variable and Implications for Natural Delivery via the Mosquito.Viruses. 2023 Sep 3;15(9):1869. doi: 10.3390/v15091869. Viruses. 2023. PMID: 37766276 Free PMC article. Review.
-
The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus.Pathog Glob Health. 2018 May;112(3):107-114. doi: 10.1080/20477724.2018.1464780. Epub 2018 May 8. Pathog Glob Health. 2018. PMID: 29737236 Free PMC article. Review.
Cited by
-
Aedes Mosquito Salivary Components and Their Effect on the Immune Response to Arboviruses.Front Cell Infect Microbiol. 2020 Aug 7;10:407. doi: 10.3389/fcimb.2020.00407. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850501 Free PMC article. Review.
-
The significance of mosquito saliva in arbovirus transmission and pathogenesis in the vertebrate host.One Health. 2023 Feb 12;16:100506. doi: 10.1016/j.onehlt.2023.100506. eCollection 2023 Jun. One Health. 2023. PMID: 37363242 Free PMC article.
-
The contribution of rodent models to the pathological assessment of flaviviral infections of the central nervous system.Arch Virol. 2012 Aug;157(8):1423-40. doi: 10.1007/s00705-012-1337-4. Epub 2012 May 17. Arch Virol. 2012. PMID: 22592957 Free PMC article. Review.
-
Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes.J Invest Dermatol. 2014 Jan;134(1):281-284. doi: 10.1038/jid.2013.251. Epub 2013 Jun 10. J Invest Dermatol. 2014. PMID: 23752041 No abstract available.
-
Discovery of mosquito saliva microRNAs during CHIKV infection.PLoS Negl Trop Dis. 2015 Jan 22;9(1):e0003386. doi: 10.1371/journal.pntd.0003386. eCollection 2015 Jan. PLoS Negl Trop Dis. 2015. PMID: 25612225 Free PMC article.
References
-
- Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. - PubMed
-
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. - PubMed
-
- Simon F, Savini H, Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med Clin North Am. 2008;92:1323–1343. - PubMed
-
- Hochedez P, Hausfater P, Jaureguiberry S, Gay F, Datry A, et al. Cases of chikungunya fever imported from the islands of the South West Indian Ocean to Paris, France. Euro Surveill. 2007;12
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

