Serum N-glycome biomarker for monitoring development of DENA-induced hepatocellular carcinoma in rat
- PMID: 20704698
- PMCID: PMC2925372
- DOI: 10.1186/1476-4598-9-215
Serum N-glycome biomarker for monitoring development of DENA-induced hepatocellular carcinoma in rat
Abstract
Background: There is a demand for serum markers for the routine assessment of the progression of liver cancer. We previously found that serum N-linked sugar chains are altered in hepatocellular carcinoma (HCC). Here, we studied glycomic alterations during development of HCC in a rat model.
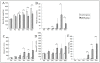
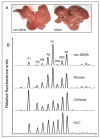
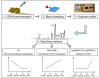
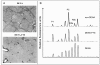
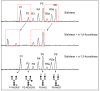
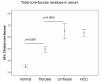
Results: Rat HCC was induced by the hepatocarcinogen, diethylnitrosamine (DENA). N-glycans were profiled using the DSA-FACE technique developed in our laboratory.In comparison with control rats, DENA rats showed a gradual but significant increase in two glycans (R5a and R5b) in serum total N-glycans during progression of liver cirrhosis and cancer, and a decrease in a biantennary glycan (P5). The log of the ratio of R5a to P1 (NGA2F) and R5b to P1 [log(R5a/P1) and log(R5b/P1)] were significantly (p < 0.0001) elevated in HCC rats, but not in rats with cirrhosis or fibrosis or in control rats. We thus propose a GlycoTest model using the above-mentioned serum glycan markers to monitor the progression of cirrhosis and HCC in the DENA-treated rat model. When DENA-treated rats were subsequently treated with farnesylthiosalicyclic acid, an anticancer drug, progression to HCC was prevented and GlycoTest markers (P5, R5a and R5b) reverted towards non-DENA levels, and the HCC-specific markers, log(R5a/P1) and log(R5b/P1), normalized completely.
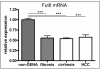
Conclusions: We found an increase in core-alpha-1,6-fucosylated glycoproteins in serum and liver of rats with HCC, which demonstrates that fucosylation is altered during progression of HCC. Our GlycoTest model can be used to monitor progression of HCC and to follow up treatment of liver tumors in the DENA rat. This GlycoTest model is particularly important because a rapid non-invasive diagnostic procedure for tumour progression in this rat model would greatly facilitate the search for anticancer drugs.
Figures



Similar articles
-
Alteration of N-glycome in diethylnitrosamine-induced hepatocellular carcinoma mice: a non-invasive monitoring tool for liver cancer.Liver Int. 2010 Sep;30(8):1221-8. doi: 10.1111/j.1478-3231.2010.02279.x. Epub 2010 May 26. Liver Int. 2010. PMID: 20524982
-
Serum serotonin as unexpected potential marker for staging of experimental hepatocellular carcinoma.Biomed Pharmacother. 2016 Oct;83:407-411. doi: 10.1016/j.biopha.2016.07.005. Epub 2016 Jul 15. Biomed Pharmacother. 2016. PMID: 27424322
-
A subnecrogenic dose of diethylnitrosamine is able to initiate hepatocarcinogenesis in the rat when coupled with fasting/refeeding.Carcinogenesis. 1996 Feb;17(2):289-92. doi: 10.1093/carcin/17.2.289. Carcinogenesis. 1996. PMID: 8625452
-
Hepatocellular carcinoma is induced by a subnecrogenic dose of diethylnitrosamine in previously fasted-refed rats.Nutr Cancer. 1998;32(1):49-54. doi: 10.1080/01635589809514716. Nutr Cancer. 1998. PMID: 9824857
-
A novel chemoprotective effect of tiopronin against diethylnitrosamine-induced hepatocellular carcinoma in rats: Role of ASK1/P38 MAPK-P53 signalling cascade.Clin Exp Pharmacol Physiol. 2020 Feb;47(2):322-332. doi: 10.1111/1440-1681.13204. Epub 2019 Nov 19. Clin Exp Pharmacol Physiol. 2020. PMID: 31663622
Cited by
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2009-2010.Mass Spectrom Rev. 2015 May-Jun;34(3):268-422. doi: 10.1002/mas.21411. Epub 2014 May 26. Mass Spectrom Rev. 2015. PMID: 24863367 Free PMC article. Review.
-
Protein and site specificity of fucosylation in liver-secreted glycoproteins.J Proteome Res. 2014 Dec 5;13(12):5561-9. doi: 10.1021/pr5005482. Epub 2014 Oct 10. J Proteome Res. 2014. PMID: 25265424 Free PMC article.
-
Curcumae Ameliorates Diethylnitrosamine-Induced Hepatocellular Carcinoma via Alteration of Oxidative Stress, Inflammation and Gut Microbiota.J Inflamm Res. 2021 Oct 27;14:5551-5566. doi: 10.2147/JIR.S330499. eCollection 2021. J Inflamm Res. 2021. PMID: 34737604 Free PMC article.
-
Cell surface-specific N-glycan profiling in breast cancer.PLoS One. 2013 Aug 23;8(8):e72704. doi: 10.1371/journal.pone.0072704. eCollection 2013. PLoS One. 2013. PMID: 24009699 Free PMC article.
-
Deciphering hepatocellular carcinoma through metabolomics: from biomarker discovery to therapy evaluation.Cancer Manag Res. 2018 Apr 11;10:715-734. doi: 10.2147/CMAR.S156837. eCollection 2018. Cancer Manag Res. 2018. PMID: 29692630 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

