Resolvin E1 regulates adenosine diphosphate activation of human platelets
- PMID: 20702811
- PMCID: PMC2982748
- DOI: 10.1161/ATVBAHA.110.209908
Resolvin E1 regulates adenosine diphosphate activation of human platelets
Abstract
Objective: To investigate the ability of resolvin E1 (RvE1) to regulate adenosine diphosphate (ADP) activation of platelets via specific receptors because RvE1 reduces platelet aggregation with certain agonists, including ADP.
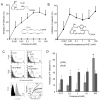
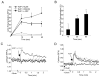
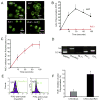
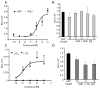
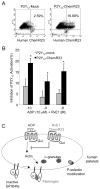
Methods and results: RvE1 is an eicosapentaenoic acid-derived specialized proresolving mediator generated during the resolution of acute inflammation. RvE1 exhibits potent organ-protective actions in vivo and acts on specific cell types, including platelets. RvE1, 0.1 to 100 nmol/L, incubated with platelets gave reduced ADP-stimulated P-selectin mobilization (IC(50), approximately 1.6×10(-12) mol/L) and polymerized actin content compared with control platelets. RvE1, 1 to 100 nmol/L, did not stimulate or block intracellular Ca(2+) mobilization. By using a new P2Y(12)-β-arrestin-coupled cell system, ADP-activated P2Y(12) with an EC(50) of 5×10(-6) mol/L and RvE1 did not directly stimulate P2Y(12) or block the ADP-P2Y(12) signals. In this system, another eicosanoid, leukotriene E(4) (LTE(4)) (EC(50), 1.3×10(-11) mol/L), dose dependently activated P2Y(12). When recombinant P2Y(12)-expressing cells were transiently transfected with an RvE1 receptor, human ChemR23 (present on human platelets), with the addition of RvE1 (0.1-10.0 nmol/L), blocked ADP signals (IC(50), approximately 1.6×10(-11) mol/L) in P2Y(12)-ChemR23-expressing cells compared with mock transfections.
Conclusions: RvE1's regulatory actions (ie, reducing ADP-stimulated P-selectin mobilization and actin polymerization) are human (h)ChemR23-dependent. Moreover, specific platelet actions of RvE1 selectively engaged with ADP-activated platelets that illuminate a new cellular mechanism and affect ω-3 eicosapentaenoic acid, which may contribute to both resolution of vascular inflammation and ADP-dependent platelet activation relevant in pathological cardiovascular events.
Figures
Similar articles
-
Rapid resensitization of purinergic receptor function in human platelets.J Thromb Haemost. 2008 Aug;6(8):1393-404. doi: 10.1111/j.1538-7836.2008.03039.x. Epub 2008 May 28. J Thromb Haemost. 2008. PMID: 18513210
-
Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets.Blood. 2008 Aug 1;112(3):848-55. doi: 10.1182/blood-2007-11-122598. Epub 2008 May 14. Blood. 2008. PMID: 18480426 Free PMC article.
-
Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation.J Immunol. 2007 Mar 15;178(6):3912-7. doi: 10.4049/jimmunol.178.6.3912. J Immunol. 2007. PMID: 17339491 Clinical Trial.
-
The P2 receptors and congenital platelet function defects.Semin Thromb Hemost. 2005 Apr;31(2):168-73. doi: 10.1055/s-2005-869522. Semin Thromb Hemost. 2005. PMID: 15852220 Review.
-
The P2 receptors in platelet function.Semin Thromb Hemost. 2005 Apr;31(2):150-61. doi: 10.1055/s-2005-869520. Semin Thromb Hemost. 2005. PMID: 15852218 Review.
Cited by
-
Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology.Circ Res. 2016 Jun 24;119(1):113-30. doi: 10.1161/CIRCRESAHA.116.307308. Circ Res. 2016. PMID: 27340271 Free PMC article. Review.
-
Blood cells: an historical account of the roles of purinergic signalling.Purinergic Signal. 2015 Dec;11(4):411-34. doi: 10.1007/s11302-015-9462-7. Epub 2015 Aug 11. Purinergic Signal. 2015. PMID: 26260710 Free PMC article. Review.
-
Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation.Am J Physiol Cell Physiol. 2013 Feb 1;304(3):C273-9. doi: 10.1152/ajpcell.00174.2012. Epub 2012 Nov 21. Am J Physiol Cell Physiol. 2013. PMID: 23174566 Free PMC article.
-
Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation.Cells. 2022 Jun 17;11(12):1957. doi: 10.3390/cells11121957. Cells. 2022. PMID: 35741086 Free PMC article. Review.
-
Inflammation and its resolution as determinants of acute coronary syndromes.Circ Res. 2014 Jun 6;114(12):1867-79. doi: 10.1161/CIRCRESAHA.114.302699. Circ Res. 2014. PMID: 24902971 Free PMC article. Review.
References
-
- Majno G, Joris I. Cells, tissues, and disease : Principles of general pathology. New York: Oxford University Press; 2004.
-
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and cotran pathologic basis of disease. Philadelphia: Elsevier/Saunders; 2005.
-
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. - PubMed
-
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. - PubMed
-
- Serhan CN. Endogenous chemical mediators in anti-inflammation and pro-resolution. Curr Med Chem Anti Inflamm Anti Allergy Agents. 2002;1:177.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

