Low abundance does not mean less importance in cysteine metabolism
- PMID: 20699647
- PMCID: PMC3115188
- DOI: 10.4161/psb.5.8.12296
Low abundance does not mean less importance in cysteine metabolism
Abstract
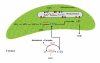
The cysteine molecule plays an essential role in cells because it is part of proteins and because it functions as a reduced sulfur donor molecule. In addition, the cysteine molecule may also play a role in the redox signaling of different stress processes. Even though the synthesis of cysteine by the most abundant of the isoforms of O-acetylserine(thiol)lyase in the chloroplast, the mitochondria and the cytosol is relatively well-understood, the role of the other less common isoforms homologous to O-acetylserine(thiol)lyase is unknown. Several studies on two of these isoforms, one located in the cytosol and the other one in the chloroplast, have shown that while one isoform operates with a desulfhydrase activity and is essential to regulate the homeostasis of cysteine in the cytosol, the other, located in the chloroplast, synthesizes S-sulfocysteine. This metabolite appears to be essential for the redox regulation of the chloroplast under certain lighting conditions.
Figures
Comment on
-
An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis.Plant Physiol. 2010 Feb;152(2):656-69. doi: 10.1104/pp.109.147975. Epub 2009 Dec 2. Plant Physiol. 2010. PMID: 19955263 Free PMC article.
-
Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control.Plant Cell. 2010 Feb;22(2):403-16. doi: 10.1105/tpc.109.071985. Epub 2010 Feb 23. Plant Cell. 2010. PMID: 20179139 Free PMC article.
Similar articles
-
S-sulfocysteine synthase function in sensing chloroplast redox status.Plant Signal Behav. 2013 Mar;8(3):e23313. doi: 10.4161/psb.23313. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333972 Free PMC article.
-
Characterization of the O-acetylserine(thiol)lyase gene family in Solanum lycopersicum L.Plant Mol Biol. 2019 Jan;99(1-2):123-134. doi: 10.1007/s11103-018-0807-9. Epub 2018 Dec 10. Plant Mol Biol. 2019. PMID: 30535734
-
The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis.Plant Cell Physiol. 2015 Feb;56(2):358-67. doi: 10.1093/pcp/pcu166. Epub 2014 Nov 20. Plant Cell Physiol. 2015. PMID: 25416292
-
Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana.Mol Plant. 2014 Feb;7(2):264-76. doi: 10.1093/mp/sst168. Epub 2013 Nov 27. Mol Plant. 2014. PMID: 24285094 Review.
-
The relevance of compartmentation for cysteine synthesis in phototrophic organisms.Protoplasma. 2012 Jun;249 Suppl 2:S147-55. doi: 10.1007/s00709-012-0411-9. Epub 2012 Apr 29. Protoplasma. 2012. PMID: 22543690 Review.
Cited by
-
Low temperature modifies seedling leaf anatomy and gene expression in Hypericum perforatum.Front Plant Sci. 2022 Sep 27;13:1020857. doi: 10.3389/fpls.2022.1020857. eCollection 2022. Front Plant Sci. 2022. PMID: 36237502 Free PMC article.
-
Genomic exploration of Sesuvium sesuvioides: comparative study and phylogenetic analysis within the order Caryophyllales from Cholistan desert, Pakistan.BMC Plant Biol. 2023 Dec 20;23(1):658. doi: 10.1186/s12870-023-04670-5. BMC Plant Biol. 2023. PMID: 38124056 Free PMC article.
-
The Functional Interplay between Ethylene, Hydrogen Sulfide, and Sulfur in Plant Heat Stress Tolerance.Biomolecules. 2022 May 8;12(5):678. doi: 10.3390/biom12050678. Biomolecules. 2022. PMID: 35625606 Free PMC article. Review.
-
Virtual 2-D map of the fungal proteome.Sci Rep. 2021 Mar 23;11(1):6676. doi: 10.1038/s41598-021-86201-6. Sci Rep. 2021. PMID: 33758316 Free PMC article.
-
Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation.Front Plant Sci. 2018 Sep 19;9:1369. doi: 10.3389/fpls.2018.01369. eCollection 2018. Front Plant Sci. 2018. PMID: 30283480 Free PMC article. Review.
References
-
- Wirtz M, Droux M, Hell R. O-acetylserine(thiol) lyase: an enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J Exp Bot. 2004;55:1785–1798. - PubMed
-
- Wirtz M, Droux M. Synthesis of the sulfur amino acids: cysteine and methionine. Photosyn Res. 2005;86:345–362. - PubMed
-
- Spadaro D, Yun BW, Spoel SH, Chu C, Wang YQ, Loake GJ. The redox switch: dynamic regulation of protein function by cysteine modifications. Physiol Plant. 2010;138:360–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
