Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity
- PMID: 20683453
- PMCID: PMC3049507
- DOI: 10.1038/jcbfm.2010.121
Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity
Abstract
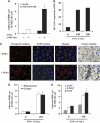
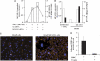
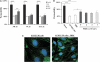
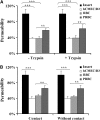
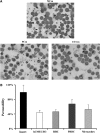
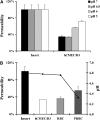
The pathogenesis of cerebral malaria (CM) remains largely unknown. There is growing evidence that combination of both parasite and host factors could be involved in blood-brain barrier (BBB) breakdown. However, lack of adequate in vitro model of human BBB so far hampered molecular studies. In this article, we propose the use of hCMEC/D3 cells, a well-established human cerebral microvascular endothelial cell (EC) line, to study BBB breakdown induced by Plasmodium falciparum-parasitized red blood cells and environmental conditions. We show that coculture of parasitized erythrocytes with hCMEC/D3 cells induces cell adhesion and paracellular permeability increase, which correlates with disorganization of zonula occludens protein 1 expression pattern. Permeability increase and modification of tight junction proteins distribution are cytoadhesion independent. Finally, we show that permeability of hCMEC/D3 cell monolayers is mediated through parasite induced metabolic acidosis, which in turns correlates with apoptosis of parasitized erythrocytes. This new coculture model represents a very useful tool, which will improve the knowledge of BBB breakdown and the development of adjuvant therapies, together with antiparasitic drugs.
Figures

Similar articles
-
Plasmodium falciparum Histidine-Rich Protein II Compromises Brain Endothelial Barriers and May Promote Cerebral Malaria Pathogenesis.mBio. 2016 Jun 7;7(3):e00617-16. doi: 10.1128/mBio.00617-16. mBio. 2016. PMID: 27273825 Free PMC article.
-
Transcriptional responses of brain endothelium to Plasmodium falciparum patient-derived isolates in vitro.Microbiol Spectr. 2024 Jul 2;12(7):e0072724. doi: 10.1128/spectrum.00727-24. Epub 2024 Jun 12. Microbiol Spectr. 2024. PMID: 38864616 Free PMC article.
-
Selection of Plasmodium falciparum parasites for cytoadhesion to human brain endothelial cells.J Vis Exp. 2012 Jan 3;(59):e3122. doi: 10.3791/3122. J Vis Exp. 2012. PMID: 22230803 Free PMC article.
-
Blood-brain barrier breakdown during cerebral malaria: suicide or murder?Thromb Haemost. 2005 Aug;94(2):336-40. doi: 10.1160/TH05-05-0354. Thromb Haemost. 2005. PMID: 16113823 Review.
-
Human cerebral malaria and the blood-brain barrier.Int J Parasitol. 2006 May 1;36(5):555-68. doi: 10.1016/j.ijpara.2006.02.004. Epub 2006 Mar 10. Int J Parasitol. 2006. PMID: 16616145 Review.
Cited by
-
Dynamic interactions of Plasmodium spp. with vascular endothelium.Tissue Barriers. 2017 Jan 2;5(1):e1268667. doi: 10.1080/21688370.2016.1268667. Epub 2016 Dec 7. Tissue Barriers. 2017. PMID: 28452684 Free PMC article. Review.
-
Specific control of pancreatic endocrine β- and δ-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9.Diabetes. 2011 Nov;60(11):2861-71. doi: 10.2337/db11-0440. Epub 2011 Sep 27. Diabetes. 2011. PMID: 21953612 Free PMC article.
-
Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it.Immunol Rev. 2020 Jan;293(1):230-252. doi: 10.1111/imr.12807. Epub 2019 Sep 27. Immunol Rev. 2020. PMID: 31562653 Free PMC article. Review.
-
Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction.Am J Pathol. 2012 Mar;180(3):1028-1039. doi: 10.1016/j.ajpath.2011.11.037. Epub 2012 Jan 17. Am J Pathol. 2012. PMID: 22260922 Free PMC article.
-
Plasmodium falciparum antigenic variation: relationships between widespread endothelial activation, parasite PfEMP1 expression and severe malaria.BMC Infect Dis. 2014 Mar 28;14:170. doi: 10.1186/1471-2334-14-170. BMC Infect Dis. 2014. PMID: 24674301 Free PMC article.
References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. - PubMed
-
- Boonpucknavig V, Boonpucknavig S, Udomsangpetch R, Nitiyanant P. An immunofluorescence study of cerebral malaria. A correlation with histopathology. Arch Pathol Lab Med. 1990;114:1028–1034. - PubMed
-
- Brown H, Hien TT, Day N, Mai NT, Chuong LV, Chau TT, Loc PP, Phu NH, Bethell D, Farrar J, Gatter K, White N, Turner G. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999a;25:331–340. - PubMed
-
- Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, Turner G. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

