CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model
- PMID: 20679726
- PMCID: PMC2929735
- DOI: 10.1172/JCI43230
CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model
Erratum in
-
CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model.J Clin Invest. 2015 Oct 1;125(10):3992. doi: 10.1172/JCI84508. Epub 2015 Oct 1. J Clin Invest. 2015. PMID: 26426080 Free PMC article. No abstract available.
Abstract
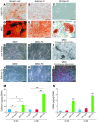
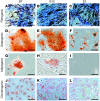
Fibroblasts are ubiquitous cells that demonstrate remarkable diversity. However, their origin and pathways of differentiation remain poorly defined. Here, we show that connective tissue growth factor (CTGF; also known as CCN2) is sufficient to induce human bone marrow mesenchymal stem/stromal cells (MSCs) to differentiate into fibroblasts. CTGF-stimulated MSCs lost their surface mesenchymal epitopes, expressed broad fibroblastic hallmarks, and increasingly synthesized collagen type I and tenacin-C. After fibroblastic commitment, the ability of MSCs to differentiate into nonfibroblastic lineages - including osteoblasts, chondrocytes, and adipocytes - was diminished. To address inherent heterogeneity in MSC culture, we established 18 single MSC-derived clones by limiting dilution. CTGF-treated MSCs were alpha-SMA-, differentiating into alpha-SMA+ myofibroblasts only when stimulated subsequently with TGF-beta1, suggestive of stepwise processes of fibroblast commitment, fibrogenesis, and pathological fibrosis. In rats, in vivo microencapsulated delivery of CTGF prompted postnatal connective tissue to undergo fibrogenesis rather than ectopic mineralization. The knowledge that fibroblasts have a mesenchymal origin may enrich our understanding of organ fibrosis, cancer stroma, ectopic mineralization, scarring, and regeneration.
Figures
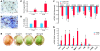


Similar articles
-
Effect of CTGF/CCN2 on osteo/cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line.J Cell Physiol. 2015 Jan;230(1):150-9. doi: 10.1002/jcp.24693. J Cell Physiol. 2015. PMID: 24905848
-
Intrinsic Deregulation of Vascular Smooth Muscle and Myofibroblast Differentiation in Mesenchymal Stromal Cells from Patients with Systemic Sclerosis.PLoS One. 2016 Apr 7;11(4):e0153101. doi: 10.1371/journal.pone.0153101. eCollection 2016. PLoS One. 2016. PMID: 27054717 Free PMC article.
-
TGF-β1 regulates differentiation of bone marrow mesenchymal stem cells.Vitam Horm. 2011;87:127-41. doi: 10.1016/B978-0-12-386015-6.00042-1. Vitam Horm. 2011. PMID: 22127241 Review.
-
In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing.Cytotherapy. 2015 Aug;17(8):1036-51. doi: 10.1016/j.jcyt.2015.04.001. Epub 2015 May 13. Cytotherapy. 2015. PMID: 25981558
-
TGF-β Family Signaling in Mesenchymal Differentiation.Cold Spring Harb Perspect Biol. 2018 May 1;10(5):a022202. doi: 10.1101/cshperspect.a022202. Cold Spring Harb Perspect Biol. 2018. PMID: 28507020 Free PMC article. Review.
Cited by
-
Harnessing endogenous stem/progenitor cells for tendon regeneration.J Clin Invest. 2015 Jul 1;125(7):2690-701. doi: 10.1172/JCI81589. Epub 2015 Jun 8. J Clin Invest. 2015. PMID: 26053662 Free PMC article.
-
Advancing application of mesenchymal stem cell-based bone tissue regeneration.Bioact Mater. 2020 Sep 21;6(3):666-683. doi: 10.1016/j.bioactmat.2020.08.014. eCollection 2021 Mar. Bioact Mater. 2020. PMID: 33005830 Free PMC article. Review.
-
Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial.Cell Transplant. 2013;22(5):767-77. doi: 10.3727/096368912X652968. Cell Transplant. 2013. PMID: 22776413 Free PMC article. Clinical Trial.
-
Matricellular proteins in cardiac adaptation and disease.Physiol Rev. 2012 Apr;92(2):635-88. doi: 10.1152/physrev.00008.2011. Physiol Rev. 2012. PMID: 22535894 Free PMC article. Review.
-
Cutaneous wound healing: recruiting developmental pathways for regeneration.Cell Mol Life Sci. 2013 Jun;70(12):2059-81. doi: 10.1007/s00018-012-1152-9. Epub 2012 Oct 4. Cell Mol Life Sci. 2013. PMID: 23052205 Free PMC article. Review.
References
-
- Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

