An engineered alpha1 integrin-binding collagenous sequence
- PMID: 20675378
- PMCID: PMC2945595
- DOI: 10.1074/jbc.M110.151357
An engineered alpha1 integrin-binding collagenous sequence
Abstract
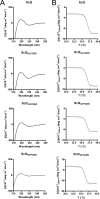
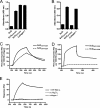
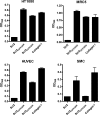
Collagen is an extracellular matrix structural component that can regulate cellular processes through its interaction with the integrins, α1β1, α2β1, α10β1, and α11β1. Collagen-like proteins have been identified in a number of bacterial species. Here, we used Scl2 from Streptococcus pyogenes serotype M28 strain MGAS6274 as a backbone for the introduction of discrete integrin-binding sequences. The introduced sequences GLPGER, GFPGER, or GFPGEN did not affect triple helix stability of the Scl (Streptococcal collagen-like) protein. Using ELISA and surface plasmon resonance, we determined that Scl2(GLPGER) and Scl2(GFPGER) bound to recombinant human α1 and α2 I-domains in a metal ion-dependent manner and without a requirement for hydroxyproline. We predicted a novel and selective integrin-binding sequence, GFPGEN, through the use of computer modeling and demonstrated that Scl2(GFPGEN) shows specificity toward the α1 I-domain and does not bind the α2 I-domain. Using C2C12 cells, we determined that intact integrins interact with the modified Scl2 proteins with the same selectivity as recombinant I-domains. These modified Scl2 proteins also acted as cell attachment substrates for fibroblast, endothelial, and smooth muscle cells. However, the modified Scl2 proteins were unable to aggregate platelets. These results indicate that Scl2 is a suitable backbone for the introduction of mammalian integrin-binding sequences, and these sequences may be manipulated to individually target α1β1 and α2β1.
Figures

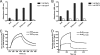


Similar articles
-
Influence of collagen-based integrin α1 and α2 mediated signaling on human mesenchymal stem cell osteogenesis in three dimensional contexts.J Biomed Mater Res A. 2018 Oct;106(10):2594-2604. doi: 10.1002/jbm.a.36451. Epub 2018 Sep 8. J Biomed Mater Res A. 2018. PMID: 29761640 Free PMC article.
-
Bioactive hydrogels based on Designer Collagens.Acta Biomater. 2010 Oct;6(10):3969-77. doi: 10.1016/j.actbio.2010.05.002. Epub 2010 May 11. Acta Biomater. 2010. PMID: 20466083
-
A streptococcal collagen-like protein interacts with the alpha2beta1 integrin and induces intracellular signaling.J Biol Chem. 2005 Apr 8;280(14):13848-57. doi: 10.1074/jbc.M410605200. Epub 2005 Jan 12. J Biol Chem. 2005. PMID: 15647274
-
The integrin-collagen connection--a glue for tissue repair?J Cell Sci. 2016 Feb 15;129(4):653-64. doi: 10.1242/jcs.180992. Epub 2016 Feb 8. J Cell Sci. 2016. PMID: 26857815 Review.
-
Integrin recognition motifs in the human collagens.Adv Exp Med Biol. 2014;819:127-42. doi: 10.1007/978-94-017-9153-3_9. Adv Exp Med Biol. 2014. PMID: 25023172 Review.
Cited by
-
Review of Integrin-Targeting Biomaterials in Tissue Engineering.Adv Healthc Mater. 2020 Dec;9(23):e2000795. doi: 10.1002/adhm.202000795. Epub 2020 Sep 16. Adv Healthc Mater. 2020. PMID: 32940020 Free PMC article. Review.
-
Design of synthetic collagens that assemble into supramolecular banded fibers as a functional biomaterial testbed.Nat Commun. 2022 Nov 9;13(1):6761. doi: 10.1038/s41467-022-34127-6. Nat Commun. 2022. PMID: 36351904 Free PMC article.
-
Adverse effects of Alport syndrome-related Gly missense mutations on collagen type IV: Insights from molecular simulations and experiments.Biomaterials. 2020 May;240:119857. doi: 10.1016/j.biomaterials.2020.119857. Epub 2020 Feb 12. Biomaterials. 2020. PMID: 32085975 Free PMC article.
-
Bacterial collagen-like proteins that form triple-helical structures.J Struct Biol. 2014 Jun;186(3):451-61. doi: 10.1016/j.jsb.2014.01.003. Epub 2014 Jan 14. J Struct Biol. 2014. PMID: 24434612 Free PMC article.
-
Influence of collagen-based integrin α1 and α2 mediated signaling on human mesenchymal stem cell osteogenesis in three dimensional contexts.J Biomed Mater Res A. 2018 Oct;106(10):2594-2604. doi: 10.1002/jbm.a.36451. Epub 2018 Sep 8. J Biomed Mater Res A. 2018. PMID: 29761640 Free PMC article.
References
-
- Leitinger B., Hohenester E. (2007) Matrix Biol. 26, 146–155 - PubMed
-
- Hulmes D. J. (1992) Essays Biochem. 27, 49–67 - PubMed
-
- Jimenez S. A., Dehm P., Olsen B. R., Prokop D. J. (1973) J. Biol. Chem. 248, 720–729 - PubMed
-
- Xu Y., Keene D. R., Bujnicki J. M., Höök M., Lukomski S. (2002) J. Biol. Chem. 277, 27312–27318 - PubMed
-
- Sylvestre P., Couture-Tosi E., Mock M. (2002) Mol. Microbiol. 45, 169–178 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

