Metabolism, excretion, and pharmacokinetics of oral brivanib in patients with advanced or metastatic solid tumors
- PMID: 20671097
- PMCID: PMC2967392
- DOI: 10.1124/dmd.110.033951
Metabolism, excretion, and pharmacokinetics of oral brivanib in patients with advanced or metastatic solid tumors
Abstract
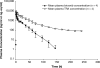
The goal of this study was to evaluate the pharmacokinetics, mass balance, metabolism, routes and extent of elimination, and safety of a single oral dose of (14)C-labeled brivanib alaninate and the safety and tolerability of brivanib after multiple doses in patients with advanced or metastatic solid tumors. This was a two-part, single-center, open-label, single oral-dose (part A) followed by multiple-dose (part B) study in patients with advanced or metastatic solid tumors. In part A, patients received a single dose of [(14)C]brivanib alaninate and in part B patients received 800 mg of nonradiolabeled brivanib alaninate every day. Four patients (two white, two black: two with non-small-cell lung cancer, one with ovarian cancer, and one with renal cell carcinoma) were treated in both parts. The median time to reach the maximal plasma concentration of brivanib was 1 h, geometric mean maximal plasma concentration was 6146 ng/ml, mean terminal half-life was 13.8 h, and geometric mean apparent oral clearance was 14.7 l/h. After a single oral dose of [(14)C]brivanib alaninate, 12.2 and 81.5% of administered radioactivity was recovered in urine and feces, respectively. Brivanib alaninate was completely converted to the active moiety, brivanib, and the predominant route of elimination was fecal. Renal excretion of unchanged brivanib was minimal. Brivanib was well tolerated; fatigue was the most frequent adverse event occurring in all patients and the most frequent treatment-related adverse event in three (75%). The best clinical response in one patient was stable disease; the other three had progressive disease. Brivanib alaninate was rapidly absorbed and extensively metabolized after a single 800-mg oral dose; the majority of drug-related radioactivity was excreted in feces.
Figures
Similar articles
-
Lack of food effect on single-dose pharmacokinetics of brivanib, and safety and efficacy following multiple doses in subjects with advanced or metastatic solid tumors.Cancer Chemother Pharmacol. 2011 Dec;68(6):1377-85. doi: 10.1007/s00280-011-1603-2. Epub 2011 Apr 3. Cancer Chemother Pharmacol. 2011. PMID: 21461891 Clinical Trial.
-
Metabolism and disposition of [14C]brivanib alaninate after oral administration to rats, monkeys, and humans.Drug Metab Dispos. 2011 May;39(5):891-903. doi: 10.1124/dmd.110.037341. Epub 2011 Feb 2. Drug Metab Dispos. 2011. PMID: 21289073
-
Preclinical pharmacokinetics and in vitro metabolism of brivanib (BMS-540215), a potent VEGFR2 inhibitor and its alanine ester prodrug brivanib alaninate.Cancer Chemother Pharmacol. 2009 Dec;65(1):55-66. doi: 10.1007/s00280-009-1002-0. Epub 2009 Apr 26. Cancer Chemother Pharmacol. 2009. PMID: 19396600
-
Lack of effect of brivanib on the pharmacokinetics of midazolam, a CYP3A4 substrate, administered intravenously and orally in healthy participants.J Clin Pharmacol. 2012 Jun;52(6):914-21. doi: 10.1177/0091270011407495. Epub 2011 Jun 9. J Clin Pharmacol. 2012. PMID: 21659627 Clinical Trial.
-
Brivanib alaninate for cancer.Expert Opin Investig Drugs. 2011 Apr;20(4):577-86. doi: 10.1517/13543784.2011.565329. Epub 2011 Mar 11. Expert Opin Investig Drugs. 2011. PMID: 21391890 Review.
Cited by
-
Targeting FGFR in Squamous Cell Carcinoma of the Lung.Target Oncol. 2017 Dec;12(6):741-755. doi: 10.1007/s11523-017-0513-6. Target Oncol. 2017. PMID: 28685321 Review.
-
FGFR1 amplifications in squamous cell carcinomas of the lung: diagnostic and therapeutic implications.Transl Lung Cancer Res. 2013 Apr;2(2):92-100. doi: 10.3978/j.issn.2218-6751.2013.03.03. Transl Lung Cancer Res. 2013. PMID: 25806220 Free PMC article. Review.
-
Pharmacokinetic Aspects of Vascular Endothelial Growth Factor Tyrosine Kinase Inhibitors.Clin Pharmacokinet. 2016 Jan;55(1):47-77. doi: 10.1007/s40262-015-0302-2. Clin Pharmacokinet. 2016. PMID: 26201307 Review.
-
Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed?Cancer Biol Ther. 2012 Mar;13(5):247-63. doi: 10.4161/cbt.19594. Cancer Biol Ther. 2012. PMID: 22481432 Free PMC article. Review.
-
Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer.Curr Cancer Drug Targets. 2012 Feb;12(2):107-23. doi: 10.2174/156800912799095144. Curr Cancer Drug Targets. 2012. PMID: 22165970 Free PMC article. Review.
References
-
- Arora A, Scholar EM. (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315:971–979 - PubMed
-
- Beumer JH, Beijnen JH, Schellens JH. (2006) Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet 45:33–58 - PubMed
-
- Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI, et al. (2006) Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem 49:2143–2146 - PubMed
-
- Bhide RS, Lombardo LJ, Hunt JT, Cai ZW, Barrish JC, Galbraith S, Jeyaseelan R, Sr, Mortillo S, Wautlet BS, Krishnan B, et al. (2010) The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther 9:369–378 - PubMed
-
- Cai ZW, Zhang Y, Borzilleri RM, Qian L, Barbosa S, Wei D, Zheng X, Wu L, Fan J, Shi Z, et al. (2008) Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215). J Med Chem 51:1976–1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources