Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes
- PMID: 20664791
- PMCID: PMC2904800
- DOI: 10.1371/journal.ppat.1001003
Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes
Erratum in
-
Correction: Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes.PLoS Pathog. 2010 Aug 10;6(8):10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. doi: 10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. PLoS Pathog. 2010. PMID: 20714345 Free PMC article.
Abstract
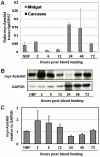
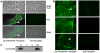
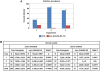
Malaria (Plasmodium spp.) kills nearly one million people annually and this number will likely increase as drug and insecticide resistance reduces the effectiveness of current control strategies. The most important human malaria parasite, Plasmodium falciparum, undergoes a complex developmental cycle in the mosquito that takes approximately two weeks and begins with the invasion of the mosquito midgut. Here, we demonstrate that increased Akt signaling in the mosquito midgut disrupts parasite development and concurrently reduces the duration that mosquitoes are infective to humans. Specifically, we found that increased Akt signaling in the midgut of heterozygous Anopheles stephensi reduced the number of infected mosquitoes by 60-99%. Of those mosquitoes that were infected, we observed a 75-99% reduction in parasite load. In homozygous mosquitoes with increased Akt signaling parasite infection was completely blocked. The increase in midgut-specific Akt signaling also led to an 18-20% reduction in the average mosquito lifespan. Thus, activation of Akt signaling reduced the number of infected mosquitoes, the number of malaria parasites per infected mosquito, and the duration of mosquito infectivity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

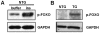
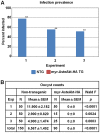
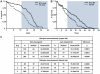
Similar articles
-
Correction: Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes.PLoS Pathog. 2010 Aug 10;6(8):10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. doi: 10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. PLoS Pathog. 2010. PMID: 20714345 Free PMC article.
-
Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi.J Exp Biol. 2013 Jan 15;216(Pt 2):208-17. doi: 10.1242/jeb.078873. J Exp Biol. 2013. PMID: 23255191 Free PMC article.
-
Inhibition of JNK signaling in the Asian malaria vector Anopheles stephensi extends mosquito longevity and improves resistance to Plasmodium falciparum infection.PLoS Pathog. 2018 Nov 29;14(11):e1007418. doi: 10.1371/journal.ppat.1007418. eCollection 2018 Nov. PLoS Pathog. 2018. PMID: 30496310 Free PMC article.
-
Interactions of human malaria parasites, Plasmodium vivax and P.falciparum, with the midgut of Anopheles mosquitoes.Med Vet Entomol. 1997 Jul;11(3):290-6. doi: 10.1111/j.1365-2915.1997.tb00409.x. Med Vet Entomol. 1997. PMID: 9330262 Review.
-
Malaria parasite development in mosquitoes.Annu Rev Entomol. 1998;43:519-43. doi: 10.1146/annurev.ento.43.1.519. Annu Rev Entomol. 1998. PMID: 9444756 Review.
Cited by
-
Converting endogenous genes of the malaria mosquito into simple non-autonomous gene drives for population replacement.Elife. 2021 Apr 13;10:e58791. doi: 10.7554/eLife.58791. Elife. 2021. PMID: 33845943 Free PMC article.
-
Insulin signaling and the regulation of insect diapause.Front Physiol. 2013 Jul 22;4:189. doi: 10.3389/fphys.2013.00189. eCollection 2013. Front Physiol. 2013. PMID: 23885240 Free PMC article.
-
The effects of ingested mammalian blood factors on vector arthropod immunity and physiology.Microbes Infect. 2013 Mar;15(3):243-54. doi: 10.1016/j.micinf.2013.01.003. Epub 2013 Jan 28. Microbes Infect. 2013. PMID: 23370408 Free PMC article. Review.
-
Integral gene drives for population replacement.Biol Open. 2019 Jan 3;8(1):bio037762. doi: 10.1242/bio.037762. Biol Open. 2019. PMID: 30498016 Free PMC article.
-
Population replacement gene drive characteristics for malaria elimination in a range of seasonal transmission settings: a modelling study.Malar J. 2022 Jul 26;21(1):226. doi: 10.1186/s12936-022-04242-2. Malar J. 2022. PMID: 35883100 Free PMC article.
References
-
- Roll Back Malaria/WHO/UNICEF. World Malaria Report 2008. World Health Organization 2008
-
- Quraishi MS, Esghi N, Faghih MA. Flight range, lengths of gonotrophic cycles, and longevity of P-32-labeled Anopheles stephensi mysorensis. J Econ Entomol. 1966;59:50–55. - PubMed
-
- Reisen WK, Aslamkhan M. A release-recapture experiment with the malaria vector, Anopheles stephensi liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behaviour. Ann Trop Med Parasitol. 1979;73:251–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

