The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry
- PMID: 20660204
- PMCID: PMC2937812
- DOI: 10.1128/JVI.00710-10
The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry
Abstract
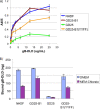
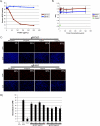
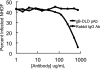
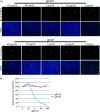
Cellular integrins were identified as human cytomegalovirus (HCMV) entry receptors and signaling mediators in both fibroblasts and endothelial cells. The goal of these studies was to determine the mechanism by which HCMV binds to cellular integrins to mediate virus entry. HCMV envelope glycoprotein B (gB) has sequence similarity to the integrin-binding disintegrin-like domain found in the ADAM (a disintegrin and metalloprotease) family of proteins. To test the ability of this region to bind to cellular integrins, we generated a recombinant soluble version of the gB disintegrin-like domain (gB-DLD). The gB-DLD protein bound to human fibroblasts in a specific, dose-dependent and saturable manner that required the expression of an intact beta1 integrin ectodomain. Furthermore, a physical association between gB-DLD and beta1 integrin was demonstrated through in vitro pull-down assays. The function of this interaction was shown by the ability of cell-bound gB-DLD to efficiently block HCMV entry and the infectivity of multiple in vivo target cells. Additionally, rabbit polyclonal antibodies raised against gB-DLD neutralized HCMV infection. Mimicry of the ADAM family disintegrin-like domain by HCMV gB represents a novel mechanism for integrin engagement by a virus and reveals a unique therapeutic target for HCMV neutralization. The strong conservation of the DLD across beta- and gammaherpesviruses suggests that integrin recognition and utilization may be a more broadly conserved feature throughout the Herpesviridae.
Figures



Similar articles
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain.Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15470-5. doi: 10.1073/pnas.0406821101. Epub 2004 Oct 19. Proc Natl Acad Sci U S A. 2004. PMID: 15494436 Free PMC article.
-
Recognition of a highly conserved glycoprotein B epitope by a bivalent antibody neutralizing HCMV at a post-attachment step.PLoS Pathog. 2020 Aug 3;16(8):e1008736. doi: 10.1371/journal.ppat.1008736. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32745149 Free PMC article.
-
Human Cytomegalovirus Glycoprotein-Initiated Signaling Mediates the Aberrant Activation of Akt.J Virol. 2020 Jul 30;94(16):e00167-20. doi: 10.1128/JVI.00167-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32493823 Free PMC article.
-
Integrins as Herpesvirus Receptors and Mediators of the Host Signalosome.Annu Rev Virol. 2016 Sep 29;3(1):215-236. doi: 10.1146/annurev-virology-110615-035618. Epub 2016 Aug 3. Annu Rev Virol. 2016. PMID: 27501260 Review.
Cited by
-
Interplay between human cytomegalovirus and intrinsic/innate host responses: a complex bidirectional relationship.Mediators Inflamm. 2012;2012:607276. doi: 10.1155/2012/607276. Epub 2012 May 31. Mediators Inflamm. 2012. PMID: 22701276 Free PMC article. Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
-
B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies.PLoS Pathog. 2011 Aug;7(8):e1002172. doi: 10.1371/journal.ppat.1002172. Epub 2011 Aug 11. PLoS Pathog. 2011. PMID: 21852946 Free PMC article.
-
Guinea pig cytomegalovirus trimer complex gH/gL/gO uses PDGFRA as universal receptor for cell fusion and entry.Virology. 2020 Sep;548:236-249. doi: 10.1016/j.virol.2020.05.012. Epub 2020 Jun 11. Virology. 2020. PMID: 32791352 Free PMC article.
-
The human cytomegalovirus decathlon: Ten critical replication events provide opportunities for restriction.Front Cell Dev Biol. 2022 Nov 25;10:1053139. doi: 10.3389/fcell.2022.1053139. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506089 Free PMC article. Review.
References
-
- Abe, E., H. Mocharla, T. Yamate, Y. Taguchi, and S. C. Manolagas. 1999. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif. Tissue Int. 64:508-515. - PubMed
-
- AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem. Biophys. Res. Commun. 166:953-959. - PubMed
-
- Adler, B., and C. Sinzger. 2009. Endothelial cells in human cytomegalovirus infection: one host cell out of many or a crucial target for virus spread? Thromb. Haemost. 102:1057-1063. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Almeida, E. A., A. P. Huovila, A. E. Sutherland, L. E. Stephens, P. G. Calarco, L. M. Shaw, A. M. Mercurio, A. Sonnenberg, P. Primakoff, D. G. Myles, et al. 1995. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81:1095-1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

