Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice
- PMID: 20659474
- PMCID: PMC4129642
- DOI: 10.1053/j.gastro.2010.07.041
Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice
Abstract
Background & aims: Alcohol abuse is a major cause of liver injury. The pathologic features of alcoholic liver disease develop over prolonged periods, yet the cellular defense mechanisms against the detrimental effects of alcohol are not well understood. We investigated whether macroautophagy, an evolutionarily conserved cellular mechanism that is commonly activated in response to stress, could protect liver cells from ethanol toxicity.
Methods: Mice were acutely given ethanol by gavage. The effects of ethanol on primary hepatocytes and hepatic cell lines were also studied in vitro.
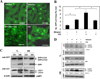
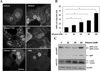
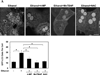
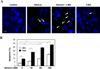
Results: Ethanol-induced macroautophagy in the livers of mice and cultured cells required ethanol metabolism, generation of reactive oxygen species, and inhibition of mammalian target of rapamycin signaling. Suppression of macroautophagy with pharmacologic agents or small interfering RNAs significantly increased hepatocyte apoptosis and liver injury; macroautophagy therefore protected cells from the toxic effects of ethanol. Macroautophagy induced by ethanol seemed to be selective for damaged mitochondria and accumulated lipid droplets, but not long-lived proteins, which could account for its protective effects. Increasing macroautophagy pharmacologically reduced hepatotoxicity and steatosis associated with acute ethanol exposure.
Conclusions: Macroautophagy protects against ethanol-induced toxicity in livers of mice. Reagents that modify macroautophagy might be developed as therapeutics for patients with alcoholic liver disease.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures













Similar articles
-
Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice.J Hepatol. 2013 May;58(5):993-9. doi: 10.1016/j.jhep.2013.01.011. Epub 2013 Jan 20. J Hepatol. 2013. PMID: 23339953 Free PMC article.
-
Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans.Gastroenterology. 2015 Oct;149(4):1030-41.e6. doi: 10.1053/j.gastro.2015.06.009. Epub 2015 Jun 20. Gastroenterology. 2015. PMID: 26099526 Free PMC article.
-
Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver.Gastroenterology. 2014 Mar;146(3):801-11. doi: 10.1053/j.gastro.2013.11.008. Epub 2013 Nov 18. Gastroenterology. 2014. PMID: 24262277 Free PMC article.
-
Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis.Semin Liver Dis. 2009 May;29(2):200-10. doi: 10.1055/s-0029-1214375. Epub 2009 Apr 22. Semin Liver Dis. 2009. PMID: 19387919 Free PMC article. Review.
-
The Activation and Function of Autophagy in Alcoholic Liver Disease.Curr Mol Pharmacol. 2017;10(3):165-171. doi: 10.2174/1874467208666150817112654. Curr Mol Pharmacol. 2017. PMID: 26278385 Free PMC article. Review.
Cited by
-
Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues.Gastroenterology. 2012 Apr;142(4):938-46. doi: 10.1053/j.gastro.2011.12.044. Epub 2012 Jan 10. Gastroenterology. 2012. PMID: 22240484 Free PMC article.
-
Autophagy and apoptosis in liver injury.Cell Cycle. 2015;14(11):1631-42. doi: 10.1080/15384101.2015.1038685. Cell Cycle. 2015. PMID: 25927598 Free PMC article. Review.
-
Regulation and Functions of Autophagic Lipolysis.Trends Endocrinol Metab. 2016 Oct;27(10):696-705. doi: 10.1016/j.tem.2016.06.003. Epub 2016 Jun 27. Trends Endocrinol Metab. 2016. PMID: 27365163 Free PMC article. Review.
-
Role of autophagy in alcohol and drug-induced liver injury.Food Chem Toxicol. 2020 Feb;136:111075. doi: 10.1016/j.fct.2019.111075. Epub 2019 Dec 23. Food Chem Toxicol. 2020. PMID: 31877367 Free PMC article. Review.
-
Hepatic NPC1L1 overexpression attenuates alcoholic autophagy in mice.Mol Med Rep. 2019 Oct;20(4):3224-3232. doi: 10.3892/mmr.2019.10549. Epub 2019 Aug 1. Mol Med Rep. 2019. PMID: 31432115 Free PMC article.
References
-
- Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. - PubMed
-
- Lumeng L, Crabb DW. Alcoholic liver disease. Curr Opin Gastroenterol. 2000;16:208–218. - PubMed
-
- Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. - PubMed
-
- Carmiel-Haggai M, Cederbaum AI, Nieto N. Binge ethanol exposure increases liver injury in obese rats. Gastroenterology. 2003;125:1818–1833. - PubMed
-
- Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

