Quantitation of "autophagic flux" in mature skeletal muscle
- PMID: 20657169
- PMCID: PMC3039739
- DOI: 10.4161/auto.6.7.12785
Quantitation of "autophagic flux" in mature skeletal muscle
Abstract
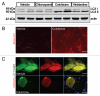
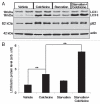
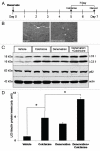
Reliable and quantitative assays to measure in vivo autophagy are essential. Currently, there are varied methods for monitoring autophagy; however, it is a challenge to measure "autophagic flux" in an in vivo model system. Conversion and subsequent degradation of the microtubule-associated protein 1 light chain 3 (MAP1-LC3/LC3) to the autophagosome associated LC3-II isoform can be evaluated by immunoblot. However, static levels of endogenous LC3-II protein may render possible misinterpretations since LC3-II levels can increase, decrease or remain unchanged in the setting of autophagic induction. Therefore, it is necessary to measure LC3-II protein levels in the presence and absence of lysomotropic agents that block the degradation of LC3-II, a technique aptly named the "autophagometer." In order to measure autophagic flux in mouse skeletal muscle, we treated animals with the microtubule depolarizing agent colchicine. Two days of 0.4 mg/kg/day intraperitoneal colchicine blocked autophagosome maturation to autolysosomes and increased LC3-II protein levels in mouse skeletal muscle by >100%. The addition of an autophagic stimulus such as dietary restriction or rapamycin led to an additional increase in LC3-II above that seen with colchicine alone. Moreover, this increase was not apparent in the absence of a "colchicine block." Using this assay, we evaluated the autophagic response in skeletal muscle upon denervation induced atrophy. Our studies highlight the feasibility of performing an "in vivo autophagometer" study using colchicine in skeletal muscle.
Figures
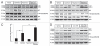
Similar articles
-
Quantification of autophagy flux using LC3 ELISA.Anal Biochem. 2017 Aug 1;530:57-67. doi: 10.1016/j.ab.2017.05.003. Epub 2017 May 4. Anal Biochem. 2017. PMID: 28477964
-
Increased autophagy accelerates colchicine-induced muscle toxicity.Autophagy. 2013 Dec;9(12):2115-25. doi: 10.4161/auto.26150. Autophagy. 2013. PMID: 24184927
-
A method to measure cardiac autophagic flux in vivo.Autophagy. 2008 Apr;4(3):322-9. doi: 10.4161/auto.5603. Epub 2008 Jan 18. Autophagy. 2008. PMID: 18216495 Free PMC article.
-
How to interpret LC3 immunoblotting.Autophagy. 2007 Nov-Dec;3(6):542-5. doi: 10.4161/auto.4600. Epub 2007 Jun 19. Autophagy. 2007. PMID: 17611390 Review.
-
Assessing autophagy in murine skeletal muscle: current findings to modulate and quantify the autophagic flux.Curr Opin Clin Nutr Metab Care. 2019 Sep;22(5):355-362. doi: 10.1097/MCO.0000000000000579. Curr Opin Clin Nutr Metab Care. 2019. PMID: 31145123 Review.
Cited by
-
Key considerations for investigating and interpreting autophagy in skeletal muscle.Autophagy. 2024 Oct;20(10):2121-2132. doi: 10.1080/15548627.2024.2373676. Epub 2024 Jul 15. Autophagy. 2024. PMID: 39007805 Review.
-
Consilience in sarcopenia of cirrhosis.J Cachexia Sarcopenia Muscle. 2012 Dec;3(4):225-37. doi: 10.1007/s13539-012-0069-3. Epub 2012 May 31. J Cachexia Sarcopenia Muscle. 2012. PMID: 22648736 Free PMC article.
-
Inclusion Body Myositis: Update on Pathogenesis and Treatment.Neurotherapeutics. 2018 Oct;15(4):995-1005. doi: 10.1007/s13311-018-0658-8. Neurotherapeutics. 2018. PMID: 30136253 Free PMC article. Review.
-
A PCR analysis of the ubiquitin-like conjugation systems in macroautophagy.Autophagy. 2011 Dec;7(12):1410-4. doi: 10.4161/auto.7.12.16991. Autophagy. 2011. PMID: 22024756 Free PMC article.
-
Ablation of Bax and Bak protects skeletal muscle against pressure-induced injury.Sci Rep. 2018 Feb 27;8(1):3689. doi: 10.1038/s41598-018-21853-5. Sci Rep. 2018. PMID: 29487339 Free PMC article.
References
-
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. - PubMed
-
- Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, et al. In search of an “autophagomometer.”. Autophagy. 2009;5:585–589. - PubMed
-
- Kuncl RW, Bilak MM, Craig SW, Adams R. Exocytotic “constipation” is a mechanism of tubulin/lysosomal interaction in colchicine myopathy. Exp Cell Res. 2003;285:196–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
