Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4
- PMID: 20650893
- PMCID: PMC2943294
- DOI: 10.1074/jbc.M110.141549
Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4
Abstract
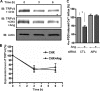
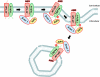
β-Arrestins, originally discovered to desensitize activated G protein-coupled receptors, (aka seven-transmembrane receptors, 7TMRs) also mediate 7TMR internalization and G protein-independent signaling via these receptors. More recently, several regulatory roles of β-arrestins for atypical 7TMRs and non-7TM receptors have emerged. Here, we uncover an entirely novel regulatory role of β-arrestins in cross-talk between the angiotensin receptor (AT1aR) and a member of the transient receptor potential (TRP) ion channel family, TRPV4. AT1aR and TRPV4 form a constitutive complex in the plasma membrane, and angiotensin stimulation leads to recruitment of β-arrestin 1 to this complex. Surprisingly, angiotensin stimulation results in ubiquitination of TRPV4, a process that requires β-arrestin 1, and subsequently to internalization and functional down-regulation of TRPV4. β-Arrestin 1 interacts with, and acts as an adaptor for AIP4, an E3 ubiquitin ligase responsible for TRPV4 ubiquitination. Thus, our data provide the first evidence of a functional link between β-arrestins and TRPV4 and uncovers an entirely novel mechanism to maintain appropriate intracellular Ca(2+) concentration to avoid excessive Ca(2+) signaling.
Figures
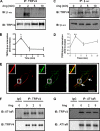
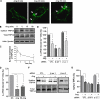
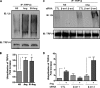
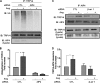
Similar articles
-
Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4.J Biol Chem. 2007 Dec 21;282(51):36971-9. doi: 10.1074/jbc.M705085200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947233
-
The nonselective cation channel TRPV4 inhibits angiotensin II receptors.J Biol Chem. 2020 Jul 17;295(29):9986-9997. doi: 10.1074/jbc.RA120.014325. Epub 2020 Jun 3. J Biol Chem. 2020. PMID: 32493776 Free PMC article.
-
Arrestins and protein ubiquitination.Prog Mol Biol Transl Sci. 2013;118:175-204. doi: 10.1016/B978-0-12-394440-5.00007-3. Prog Mol Biol Transl Sci. 2013. PMID: 23764054 Review.
-
Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6650-5. doi: 10.1073/pnas.0901083106. Epub 2009 Apr 10. Proc Natl Acad Sci U S A. 2009. PMID: 19363159 Free PMC article.
-
Ubiquitin-Related Roles of β-Arrestins in Endocytic Trafficking and Signal Transduction.J Cell Physiol. 2016 Oct;231(10):2071-80. doi: 10.1002/jcp.25317. Epub 2016 Feb 3. J Cell Physiol. 2016. PMID: 26790995 Review.
Cited by
-
Differential ubiquitination and proteasome regulation of Ca(V)2.2 N-type channel splice isoforms.J Neurosci. 2012 Jul 25;32(30):10365-9. doi: 10.1523/JNEUROSCI.0851-11.2012. J Neurosci. 2012. PMID: 22836269 Free PMC article.
-
ARRB1-Promoted NOTCH1 Degradation Is Suppressed by OncomiR miR-223 in T-cell Acute Lymphoblastic Leukemia.Cancer Res. 2020 Mar 1;80(5):988-998. doi: 10.1158/0008-5472.CAN-19-1471. Epub 2019 Dec 10. Cancer Res. 2020. PMID: 31822496 Free PMC article.
-
Role of TRP ion channels in pruritus.Neurosci Lett. 2022 Jan 18;768:136379. doi: 10.1016/j.neulet.2021.136379. Epub 2021 Nov 30. Neurosci Lett. 2022. PMID: 34861341 Free PMC article. Review.
-
Targeting Hedgehog Signalling through the Ubiquitylation Process: The Multiple Roles of the HECT-E3 Ligase Itch.Cells. 2019 Jan 29;8(2):98. doi: 10.3390/cells8020098. Cells. 2019. PMID: 30699938 Free PMC article. Review.
-
Itch/β-arrestin2-dependent non-proteolytic ubiquitylation of SuFu controls Hedgehog signalling and medulloblastoma tumorigenesis.Nat Commun. 2018 Mar 7;9(1):976. doi: 10.1038/s41467-018-03339-0. Nat Commun. 2018. PMID: 29515120 Free PMC article.
References
-
- Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. (2002) FEBS Lett. 520, 97–101 - PubMed
-
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Nat. Rev. Mol. Cell Biol. 3, 639–650 - PubMed
-
- Lefkowitz R. J. (1998) J. Biol. Chem. 273, 18677–18680 - PubMed
-
- Pitcher J. A., Freedman N. J., Lefkowitz R. J. (1998) Annu. Rev. Biochem. 67, 653–692 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

