Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1
- PMID: 20649536
- PMCID: PMC5585781
- DOI: 10.1111/j.1749-6632.2010.05629.x
Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1
Abstract
Mitochondria in cells comprise a tubulovesicular network shaped continuously by complementary fission and fusion events. The mammalian Drp1 protein plays a key role in fission, while Mfn1, Mfn2, and OPA1 are required for fusion. Shifts in the balance between these opposing processes can occur rapidly, indicating that modifications to these proteins may regulate mitochondrial membrane dynamics. We highlight posttranslational modifications of the mitochondrial fission protein Drp1, for which these regulatory mechanisms are best characterized. This dynamin-related GTPase undergoes a number of steps to mediate mitochondrial fission, including translocation from cytoplasm to the mitochondrial outer membrane, higher-order assembly into spirals, GTP hydrolysis associated with a conformational change and membrane deformation, and ultimately disassembly. Many of these steps may be influenced by covalent modification of Drp1. We discuss the dynamic nature of Drp1 modifications and how they contribute not only to the normal regulation of mitochondrial division, but also to neuropathologic processes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
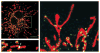
Similar articles
-
New insights into the function and regulation of mitochondrial fission.Biochim Biophys Acta. 2013 May;1833(5):1256-68. doi: 10.1016/j.bbamcr.2013.02.002. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434681 Review.
-
Specific interaction with cardiolipin triggers functional activation of Dynamin-Related Protein 1.PLoS One. 2014 Jul 18;9(7):e102738. doi: 10.1371/journal.pone.0102738. eCollection 2014. PLoS One. 2014. PMID: 25036098 Free PMC article.
-
The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction.J Biol Chem. 2015 May 1;290(18):11692-703. doi: 10.1074/jbc.M114.610881. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770210 Free PMC article.
-
Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission.EMBO J. 2011 Jun 24;30(14):2762-78. doi: 10.1038/emboj.2011.198. EMBO J. 2011. PMID: 21701560 Free PMC article.
-
[Mechanism of mitochondrial fission - structure and function of Drp1 protein].Postepy Biochem. 2016;62(2):127-137. Postepy Biochem. 2016. PMID: 28132464 Review. Polish.
Cited by
-
Molecular mechanisms of mitochondrial dynamics.Nat Rev Mol Cell Biol. 2024 Oct 17. doi: 10.1038/s41580-024-00785-1. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39420231 Review.
-
Podocyte-specific KLF6 primes proximal tubule CaMK1D signaling to attenuate diabetic kidney disease.Nat Commun. 2024 Sep 13;15(1):8038. doi: 10.1038/s41467-024-52306-5. Nat Commun. 2024. PMID: 39271683 Free PMC article.
-
The multifaceted role of mitochondria in autism spectrum disorder.Mol Psychiatry. 2024 Sep 2. doi: 10.1038/s41380-024-02725-z. Online ahead of print. Mol Psychiatry. 2024. PMID: 39223276 Review.
-
Pim-1 kinase protects the liver from ischemia reperfusion injury by regulating dynamics-related protein 1.iScience. 2024 Jun 28;27(7):110280. doi: 10.1016/j.isci.2024.110280. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055921 Free PMC article.
-
ROS-mediated cytoplasmic localization of CARM1 induces mitochondrial fission through DRP1 methylation.Redox Biol. 2024 Jul;73:103212. doi: 10.1016/j.redox.2024.103212. Epub 2024 May 31. Redox Biol. 2024. PMID: 38838552 Free PMC article.
References
-
- Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. - PubMed
-
- Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. 2009;1793:154–170. - PubMed
-
- Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

