Convergent transcription through a long CAG tract destabilizes repeats and induces apoptosis
- PMID: 20647539
- PMCID: PMC2937530
- DOI: 10.1128/MCB.00332-10
Convergent transcription through a long CAG tract destabilizes repeats and induces apoptosis
Abstract
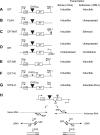
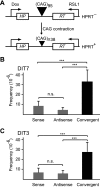
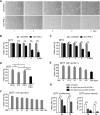
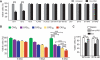
Short repetitive sequences are common in the human genome, and many fall within transcription units. We have previously shown that transcription through CAG repeat tracts destabilizes them in a way that depends on transcription-coupled nucleotide excision repair and mismatch repair. Recent observations that antisense transcription accompanies sense transcription in many human genes led us to test the effects of antisense transcription on triplet repeat instability in human cells. Here, we report that simultaneous sense and antisense transcription (convergent transcription) initiated from two inducible promoters flanking a CAG95 tract in a nonessential gene enhances repeat instability synergistically, arrests the cell cycle, and causes massive cell death via apoptosis. Using chemical inhibitors and small interfering RNA (siRNA) knockdowns, we identified the ATR (ataxia-telangiectasia mutated [ATM] and Rad3 related) signaling pathway as a key mediator of this cellular response. RNA polymerase II, replication protein A (RPA), and components of the ATR signaling pathway accumulate at convergently transcribed repeat tracts, accompanied by phosphorylation of ATR, CHK1, and p53. Cell death depends on simultaneous sense and antisense transcription and is proportional to their relative levels, it requires the presence of the repeat tract, and it occurs in both proliferating and nonproliferating cells. Convergent transcription through a CAG repeat represents a novel mechanism for triggering a cellular stress response, one that is initiated by events at a single locus in the genome and resembles the response to DNA damage.
Figures
Similar articles
-
ATR and Chk1 suppress a caspase-3-dependent apoptotic response following DNA replication stress.PLoS Genet. 2009 Jan;5(1):e1000324. doi: 10.1371/journal.pgen.1000324. Epub 2009 Jan 2. PLoS Genet. 2009. PMID: 19119425 Free PMC article.
-
Ultrasound activates ataxia telangiectasia mutated- and rad3-related (ATR)-checkpoint kinase 1 (Chk1) pathway in human leukemia Jurkat cells.Ultrason Sonochem. 2012 Nov;19(6):1246-51. doi: 10.1016/j.ultsonch.2012.04.003. Epub 2012 Apr 19. Ultrason Sonochem. 2012. PMID: 22571845
-
Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA, and CHK1.Genes Dev. 2008 Mar 1;22(5):587-600. doi: 10.1101/gad.1627708. Epub 2008 Feb 18. Genes Dev. 2008. PMID: 18283122 Free PMC article.
-
Dual regulation of Cdc25A by Chk1 and p53-ATF3 in DNA replication checkpoint control.J Biol Chem. 2009 Feb 13;284(7):4132-9. doi: 10.1074/jbc.M808118200. Epub 2008 Dec 7. J Biol Chem. 2009. PMID: 19060337
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
Cited by
-
All three MutL complexes are required for repeat expansion in a human stem cell model of CAG-repeat expansion mediated glutaminase deficiency.bioRxiv [Preprint]. 2024 May 9:2023.12.26.573357. doi: 10.1101/2023.12.26.573357. bioRxiv. 2024. Update in: Sci Rep. 2024 Jun 14;14(1):13772. doi: 10.1038/s41598-024-64480-z. PMID: 38260514 Free PMC article. Updated. Preprint.
-
Oligodeoxynucleotide binding to (CTG) · (CAG) microsatellite repeats inhibits replication fork stalling, hairpin formation, and genome instability.Mol Cell Biol. 2013 Feb;33(3):571-81. doi: 10.1128/MCB.01265-12. Epub 2012 Nov 19. Mol Cell Biol. 2013. PMID: 23166299 Free PMC article.
-
GFP-based fluorescence assay for CAG repeat instability in cultured human cells.PLoS One. 2014 Nov 25;9(11):e113952. doi: 10.1371/journal.pone.0113952. eCollection 2014. PLoS One. 2014. PMID: 25423602 Free PMC article.
-
Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1.Hum Mol Genet. 2011 Dec 15;20(24):4822-30. doi: 10.1093/hmg/ddr421. Epub 2011 Sep 15. Hum Mol Genet. 2011. PMID: 21926083 Free PMC article.
-
Splice modulators target PMS1 to reduce somatic expansion of the Huntington's disease-associated CAG repeat.Nat Commun. 2024 Apr 12;15(1):3182. doi: 10.1038/s41467-024-47485-0. Nat Commun. 2024. PMID: 38609352 Free PMC article.
References
-
- Arima, Y., M. Nitta, S. Kuninaka, D. Zhang, T. Fujiwara, Y. Taya, M. Nakao, and H. Saya. 2005. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J. Biol. Chem. 280:19166-19176. - PubMed
-
- Belotserkovskii, B. P., E. De Silva, S. Tornaletti, G. Wang, K. M. Vasquez, and P. C. Hanawalt. 2007. A triplex-forming sequence from the human c-MYC promoter interferes with DNA transcription. J. Biol. Chem. 282:32433-32441. - PubMed
-
- Chen, W. L., J. W. Lin, H. J. Huang, S. M. Wang, M. T. Su, G. J. Lee-Chen, C. M. Chen, and H. M. Hsieh-Li. 2008. SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res. 1233:176-184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
