Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress
- PMID: 20647536
- PMCID: PMC2950528
- DOI: 10.1128/MCB.00657-10
Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress
Abstract
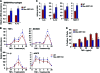
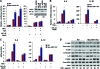
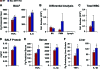
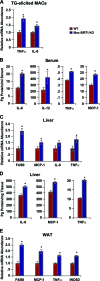
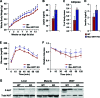
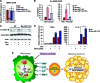
Macrophage activation and infiltration into resident tissues is known to mediate local inflammation and is a hallmark feature of metabolic syndrome. Members of the sirtuin family of proteins regulate numerous physiological processes, including those involved in nutrient regulation and the promotion of longevity. However, the important role that SIRT1, the leading sirtuin family member, plays in immune response remains unclear. In this study, we demonstrate that SIRT1 modulates the acetylation status of the RelA/p65 subunit of NF-κB and thus plays a pivotal role in regulating the inflammatory, immune, and apoptotic responses in mammals. Using a myeloid cell-specific SIRT1 knockout (Mac-SIRT1 KO) mouse model, we show that ablation of SIRT1 in macrophages renders NF-κB hyperacetylated, resulting in increased transcriptional activation of proinflammatory target genes. Consistent with increased proinflammatory gene expression, Mac-SIRT1 KO mice challenged with a high-fat diet display high levels of activated macrophages in liver and adipose tissue, predisposing the animals to development of systemic insulin resistance and metabolic derangement. In summary, we report that SIRT1, in macrophages, functions to inhibit NF-κB-mediated transcription, implying that myeloid cell-specific modulation of this sirtuin may be beneficial in the treatment of inflammation and its associated diseases.
Figures

Similar articles
-
Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation.PLoS One. 2014 Feb 3;9(2):e87733. doi: 10.1371/journal.pone.0087733. eCollection 2014. PLoS One. 2014. PMID: 24498364 Free PMC article.
-
Myeloid SIRT1 regulates macrophage infiltration and insulin sensitivity in mice fed a high-fat diet.J Endocrinol. 2015 Feb;224(2):109-18. doi: 10.1530/JOE-14-0527. Epub 2014 Oct 27. J Endocrinol. 2015. PMID: 25349250
-
ROR alpha protects against LPS-induced inflammation by down-regulating SIRT1/NF-kappa B pathway.Arch Biochem Biophys. 2019 Jun 15;668:1-8. doi: 10.1016/j.abb.2019.05.003. Epub 2019 May 6. Arch Biochem Biophys. 2019. PMID: 31071300
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
-
Sirtuin-1 in immunotherapy: A Janus-headed target.J Leukoc Biol. 2019 Aug;106(2):337-343. doi: 10.1002/JLB.2RU1118-422R. Epub 2019 Jan 3. J Leukoc Biol. 2019. PMID: 30605226 Free PMC article. Review.
Cited by
-
Modulators of HIF1α and NFkB in Cancer Treatment: Is it a Rational Approach for Controlling Malignant Progression?Front Pharmacol. 2013 Feb 12;4:13. doi: 10.3389/fphar.2013.00013. eCollection 2013. Front Pharmacol. 2013. PMID: 23408731 Free PMC article.
-
SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice.J Clin Invest. 2012 Jun;122(6):2032-45. doi: 10.1172/JCI60132. Epub 2012 May 1. J Clin Invest. 2012. PMID: 22546858 Free PMC article.
-
Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):899-904. doi: 10.1073/pnas.1118462109. Epub 2012 Jan 4. Proc Natl Acad Sci U S A. 2012. PMID: 22219356 Free PMC article.
-
Dietary curcumin supplementation does not alter peripheral blood mononuclear cell responses to exertional heat stress.Eur J Appl Physiol. 2018 Dec;118(12):2707-2717. doi: 10.1007/s00421-018-3998-5. Epub 2018 Oct 1. Eur J Appl Physiol. 2018. PMID: 30276476 Clinical Trial.
-
Sirtuin-1 regulation of mammalian metabolism.Trends Mol Med. 2011 Jan;17(1):8-13. doi: 10.1016/j.molmed.2010.09.005. Trends Mol Med. 2011. PMID: 20971038 Free PMC article. Review.
References
-
- Anastasiou, D., and W. Krek. 2006. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology (Bethesda) 21:404-410. - PubMed
-
- Bäckesjä, C. M., Y. Li, U. Lindgren, and L. A. Haldosen. 2006. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J. Bone Miner. Res. 21:993-1002. - PubMed
-
- Reference deleted.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
