Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells
- PMID: 20643772
- PMCID: PMC2988518
- DOI: 10.1113/jphysiol.2010.194035
Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells
Abstract
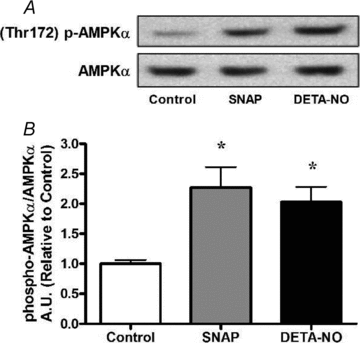
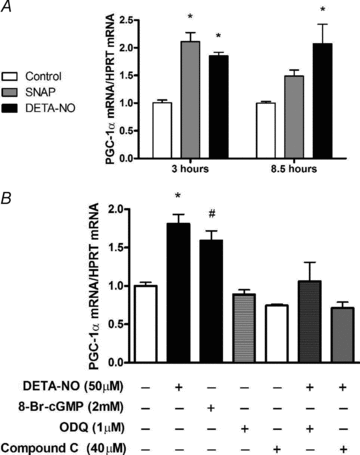
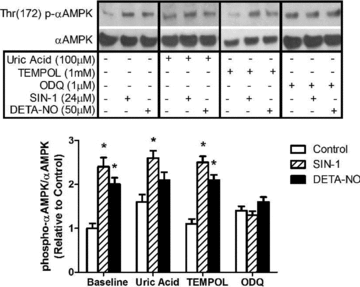
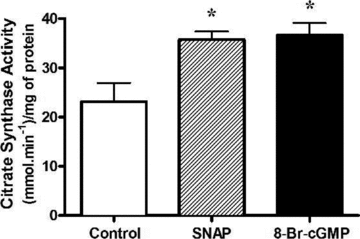
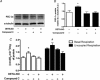
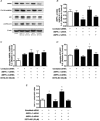
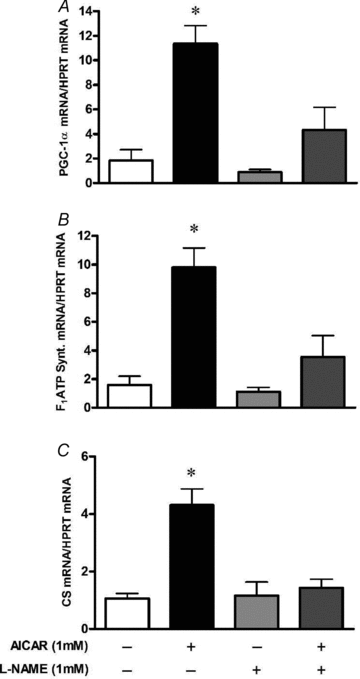
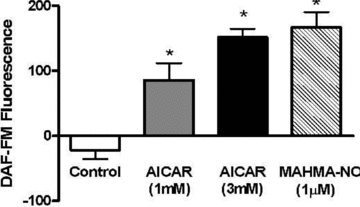
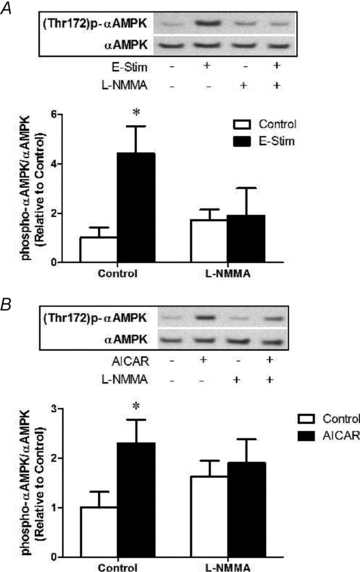
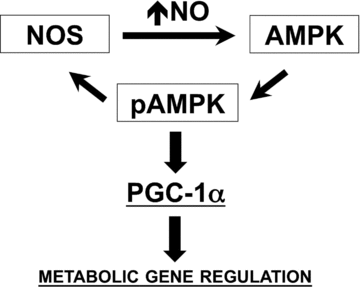
Nitric oxide (NO) induces mitochondrial biogenesis in skeletal muscle cells via upregulation of the peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α). Further, we have shown that nitric oxide interacts with the metabolic sensor enzyme, AMPK. Therefore, we tested the hypothesis that nitric oxide and AMPK act synergistically to upregulate PGC-1α mRNA expression and stimulate mitochondrial biogenesis in culture. L6 myotubes treated with nitric oxide donors, S-nitroso-N-penicillamine (SNAP, 25 μM) or diethylenetriamine-NONO (DETA-NO, 50 μM), exhibited elevated AMPK phosphorylation, PGC-1α mRNA and protein, and basal and uncoupled mitochondrial respiration (P < 0.05). Pre-treatment of cultures with the AMPK inhibitor, Compound C, prevented these effects. Knockdown of AMPKα1 in L6 myotubes using siRNA reduced AMPKα protein content and prevented upregulation of PGC-1α mRNA by DETA-NO. Meanwhile, siRNA knockdown of AMPKα2 had no effect on total AMPKα protein content or PGC-1α mRNA. These results suggest that NO effects on PGC-1α expression are mediated by AMPKα1. Paradoxically, we found that the AMPK-activating compound, AICAR, induced NO release from L6 myotubes, and that AICAR-induced upregulation of PGC-1α mRNA was prevented by inhibition of NOS with N(G)-nitro-L-arginine methyl ester (L-NAME, 1 mM). Additionally, incubation of isolated mouse extensor digitorum longus (EDL) muscles with 2 mM AICAR for 20 min or electrical stimulation (10 Hz, 13 V) for 10 min induced phosphorylation of AMPKα (P < 0.05), which was completely prevented by pre-treatment with the NOS inhibitor, L-N(G)-monomethyl arginine (L-NMMA, 1 mM). These data identify the AMPKα1 isoform as the mediator of NO-induced effects in skeletal muscle cells. Further, this study supports a proposed model of synergistic interaction between AMPK and NOS that is critical for maintenance of metabolic function in skeletal muscle cells.
Figures
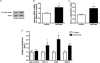
Similar articles
-
Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes.J Appl Physiol (1985). 2010 Mar;108(3):589-95. doi: 10.1152/japplphysiol.00377.2009. Epub 2009 Dec 31. J Appl Physiol (1985). 2010. PMID: 20044477
-
Role of the α2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells.Metabolism. 2019 Jan;90:52-68. doi: 10.1016/j.metabol.2018.10.003. Epub 2018 Oct 23. Metabolism. 2019. PMID: 30359677
-
Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells.Am J Physiol Cell Physiol. 2009 Jan;296(1):C116-23. doi: 10.1152/ajpcell.00267.2007. Epub 2008 Nov 12. Am J Physiol Cell Physiol. 2009. PMID: 19005163
-
Impact of fasting on the AMPK and PGC-1α axis in rodent and human skeletal muscle: A systematic review.Metabolism. 2024 Mar;152:155768. doi: 10.1016/j.metabol.2023.155768. Epub 2023 Dec 27. Metabolism. 2024. PMID: 38154612 Review.
-
Phytochemicals regulate cancer metabolism through modulation of the AMPK/PGC-1α signaling pathway.BMC Cancer. 2024 Sep 2;24(1):1079. doi: 10.1186/s12885-024-12715-7. BMC Cancer. 2024. PMID: 39223494 Free PMC article. Review.
Cited by
-
PGC-1α activity and mitochondrial dysfunction in preterm infants.Front Physiol. 2022 Sep 26;13:997619. doi: 10.3389/fphys.2022.997619. eCollection 2022. Front Physiol. 2022. PMID: 36225305 Free PMC article. Review.
-
The β3 Adrenergic Receptor Agonist CL316243 Ameliorates the Metabolic Abnormalities of High-Fat Diet-Fed Rats by Activating AMPK/PGC-1α Signaling in Skeletal Muscle.Diabetes Metab Syndr Obes. 2021 Mar 18;14:1233-1241. doi: 10.2147/DMSO.S297351. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 33776460 Free PMC article.
-
Transcription Factor Movement and Exercise-Induced Mitochondrial Biogenesis in Human Skeletal Muscle: Current Knowledge and Future Perspectives.Int J Mol Sci. 2022 Jan 28;23(3):1517. doi: 10.3390/ijms23031517. Int J Mol Sci. 2022. PMID: 35163441 Free PMC article. Review.
-
Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2022 Jun 30;23(13):7280. doi: 10.3390/ijms23137280. Int J Mol Sci. 2022. PMID: 35806284 Free PMC article. Review.
-
Protective effect of surface-modified berberine nanoparticles against LPS-induced neurodegenerative changes: a preclinical study.Drug Deliv Transl Res. 2019 Oct;9(5):906-919. doi: 10.1007/s13346-019-00626-1. Drug Deliv Transl Res. 2019. PMID: 30868509
References
-
- Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-γ coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol. 2004;287:C790–C796. - PubMed
-
- Balon TW, Jasman AP. Acute exposure to AICAR increases glucose transport in mouse EDL and soleus muscle. Biochem Biophys Res Commun. 2001;282:1008–1011. - PubMed
-
- Bonen A, Clark MG, Henriksen EJ. Experimental approaches in muscle metabolism: hindlimb perfusion and isolated muscle incubations. Am J Physiol Endocrinol Metab. 1994;266:E1–E16. - PubMed
-
- Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. - PubMed
-
- Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress via the transcriptional coactivator PGC1α. FASEB J. 2006;20:1889–1891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

