Role of transforming growth factor Beta in corneal function, biology and pathology
- PMID: 20642439
- PMCID: PMC3048459
- DOI: 10.2174/1566524011009060565
Role of transforming growth factor Beta in corneal function, biology and pathology
Abstract
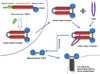
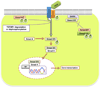
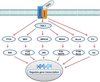
Transforming growth factor-beta (TGFbeta) is a pleiotropic multifunctional cytokine that regulates several essential cellular processes in many parts of the body including the cornea. Three isoforms of TGFbeta are known in mammals and the human cornea expresses all of them. TGFbeta1 has been shown to play a central role in scar formation in adult corneas whereas TGFbeta2 and TGFbeta3 have been implicated to play a critical role in corneal development and scarless wound healing during embryogenesis. The biological effects of TGFbeta in the cornea have been shown to follow Smad dependent as well as Smad-independent signaling pathways depending upon cellular responses and microenvironment. Corneal TGFbeta expression is necessary for maintaining corneal integrity and corneal wound healing. On the other hand, TGFbeta is perhaps the most important cytokine in the pathogenesis of fibrotic disease in the cornea. Although the transformation of keratocytes to myofibroblasts induced by TGFbeta is largely believed to cause corneal fibrosis or scarring, the precise molecular mechanism(s) involved in this process is still unknown. Currently no drugs are available to treat corneal scarring effectively without causing significant side effects. Many approaches to treat TGFbeta-mediated corneal scarring are under investigation. These include blocking of TGFbeta, TGFbeta receptor, TGFbeta function and/or TGFbeta maturation. Other strategies such as modulating keratocyte proliferation, apoptosis, transcription and DNA condensation are also being investigated. The potential of gene therapy to neutralize the pathologic effects of TGFbeta has also been demonstrated recently.
Figures




Similar articles
-
Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury.Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):8. doi: 10.1167/iovs.62.4.8. Invest Ophthalmol Vis Sci. 2021. PMID: 33825855 Free PMC article.
-
Modulation of Smad signaling by non-TGFβ components in myofibroblast generation during wound healing in corneal stroma.Exp Eye Res. 2016 Jan;142:40-8. doi: 10.1016/j.exer.2014.12.015. Exp Eye Res. 2016. PMID: 26675402 Review.
-
Decorin biology, expression, function and therapy in the cornea.Curr Mol Med. 2011 Mar;11(2):110-28. doi: 10.2174/156652411794859241. Curr Mol Med. 2011. PMID: 21342131 Review.
-
Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK.Curr Eye Res. 1998 Jul;17(7):736-47. Curr Eye Res. 1998. PMID: 9678420
-
Growth factor expression in corneal wound healing after excimer laser keratectomy.Cornea. 1999 Sep;18(5):580-8. Cornea. 1999. PMID: 10487433
Cited by
-
Distribution and Function of Glycosaminoglycans and Proteoglycans in the Development, Homeostasis and Pathology of the Ocular Surface.Front Cell Dev Biol. 2020 Aug 7;8:731. doi: 10.3389/fcell.2020.00731. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32903857 Free PMC article. Review.
-
Potential therapeutic effects of peroxisome proliferator-activated receptors on corneal diseases.Exp Biol Med (Maywood). 2024 Jun 27;249:10142. doi: 10.3389/ebm.2024.10142. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 38993197 Free PMC article. Review.
-
Hyperstable EGF-like bleogen derived from cactus accelerates corneal healing in rats.Front Pharmacol. 2022 Aug 16;13:942168. doi: 10.3389/fphar.2022.942168. eCollection 2022. Front Pharmacol. 2022. PMID: 36052138 Free PMC article.
-
BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo.PLoS One. 2013 Jun 14;8(6):e66434. doi: 10.1371/journal.pone.0066434. Print 2013. PLoS One. 2013. PMID: 23799103 Free PMC article.
-
Localization of thrombospondin-1 and myofibroblasts during corneal wound repair.Exp Eye Res. 2011 Oct;93(4):534-40. doi: 10.1016/j.exer.2011.06.018. Epub 2011 Jul 2. Exp Eye Res. 2011. PMID: 21749870 Free PMC article.
References
-
- Chin D, Boyle GM, Parsons PG, Coman WB. Br J Plast Surg. 2004;57:215–221. - PubMed
-
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. J. Biol. Chem. 1983;258:7155–7160. - PubMed
-
- Jester JV, Barry Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Cornea. 1997;16:177–187. - PubMed
-
- Saika S. Cornea. 2004;23:25–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
