Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression
- PMID: 20637660
- PMCID: PMC2914224
- DOI: 10.1016/j.immuni.2010.07.001
Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression
Abstract
Transcriptional regulation of the Nos2 gene encoding inducible nitric oxide synthase (iNOS) requires type I interferon (IFN-I) signaling and additional signals emanating from pattern recognition receptors. Here we showed sequential and cooperative contributions of the transcription factors ISGF3 (a complex containing STAT1, STAT2, and IRF9 subunits) and NF-kappaB to the transcriptional induction of the Nos2 gene in macrophages infected with the intracellular bacterial pathogen Listeria monocytogenes. NF-kappaB preceded ISGF3 at the Nos2 promoter and generated a transcriptional memory effect by depositing basal transcription factor TFIIH with the associated CDK7 kinase for serine 5 phosphorylation of the RNA polymerase II (pol II) carboxyterminal domain (CTD). Subsequent to TFIIH deposition by NF-kappaB, ISGF3 attracted the pol II enzyme and phosphorylation at CTD S5 occurred. Thus, STATs and NF-kappaB cooperate through pol II promoter recruitment and the phosphorylation of its CTD, respectively, as a prerequisite for productive elongation of iNOS mRNA.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
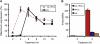
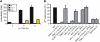
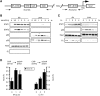
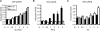
Comment in
-
NF-kappaB-ISGF3 transcription factor cooperation: coincidence detector or memory chip?Immunity. 2010 Jul 23;33(1):1-2. doi: 10.1016/j.immuni.2010.07.011. Immunity. 2010. PMID: 20643331
Similar articles
-
Cooperative Transcriptional Activation of Antimicrobial Genes by STAT and NF-κB Pathways by Concerted Recruitment of the Mediator Complex.Cell Rep. 2015 Jul 14;12(2):300-12. doi: 10.1016/j.celrep.2015.06.021. Epub 2015 Jul 2. Cell Rep. 2015. PMID: 26146080 Free PMC article.
-
Regulation of NO synthesis, local inflammation, and innate immunity to pathogens by BET family proteins.Mol Cell Biol. 2014 Feb;34(3):415-27. doi: 10.1128/MCB.01353-13. Epub 2013 Nov 18. Mol Cell Biol. 2014. PMID: 24248598 Free PMC article.
-
The role of NF-kappaB, IRF-1, and STAT-1alpha transcription factors in the iNOS gene induction by gliadin and IFN-gamma in RAW 264.7 macrophages.J Mol Med (Berl). 2006 Jan;84(1):65-74. doi: 10.1007/s00109-005-0713-x. Epub 2005 Nov 12. J Mol Med (Berl). 2006. PMID: 16284791
-
Requirement for endogenous heat shock factor 1 in inducible nitric oxide synthase induction in murine microglia.J Neuroinflammation. 2015 Oct 14;12:189. doi: 10.1186/s12974-015-0406-5. J Neuroinflammation. 2015. PMID: 26467650 Free PMC article.
-
Rotavirus and reovirus modulation of the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):559-67. doi: 10.1089/jir.2009.0072. J Interferon Cytokine Res. 2009. PMID: 19694545 Free PMC article. Review.
Cited by
-
Mechanisms of Jak/STAT signaling in immunity and disease.J Immunol. 2015 Jan 1;194(1):21-7. doi: 10.4049/jimmunol.1401867. J Immunol. 2015. PMID: 25527793 Free PMC article. Review.
-
c-Rel: shaping CD4 regulatory T cell development in unexpected ways.Transcription. 2012 Sep-Oct;3(5):245-9. doi: 10.4161/trns.21309. Epub 2012 Sep 1. Transcription. 2012. PMID: 22885978 Free PMC article.
-
Leukaemia inhibitory factor stimulates proliferation of olfactory neuronal progenitors via inducible nitric oxide synthase.PLoS One. 2012;7(9):e45018. doi: 10.1371/journal.pone.0045018. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024784 Free PMC article.
-
Nitric Oxide Engages an Anti-inflammatory Feedback Loop Mediated by Peroxiredoxin 5 in Phagocytes.Cell Rep. 2018 Jul 24;24(4):838-850. doi: 10.1016/j.celrep.2018.06.081. Cell Rep. 2018. PMID: 30044981 Free PMC article.
-
Oxygen Tension Strongly Influences Metabolic Parameters and the Release of Interleukin-6 of Human Amniotic Mesenchymal Stromal Cells In Vitro.Stem Cells Int. 2018 Oct 28;2018:9502451. doi: 10.1155/2018/9502451. eCollection 2018. Stem Cells Int. 2018. PMID: 30510589 Free PMC article.
References
-
- Baccarini M., Bistoni F., Lohmann Matthes M.L. In vitro natural cell-mediated cytotoxicity against Candida albicans: Macrophage precursors as effector cells. J. Immunol. 1985;134:2658–2665. - PubMed
-
- Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. - PubMed
-
- Chapman R.D., Heidemann M., Hintermair C., Eick D. Molecular evolution of the RNA polymerase II CTD. Trends Genet. 2008;24:289–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

