Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35
- PMID: 20631177
- PMCID: PMC2945303
- DOI: 10.1523/JNEUROSCI.4466-09.2010
Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35
Abstract
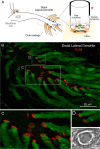
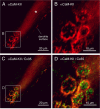
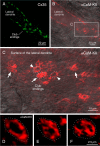
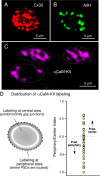
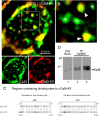
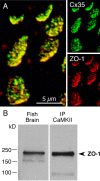
In contrast to chemical transmission, few proteins have been shown associated with gap junction-mediated electrical synapses. Mixed (electrical and glutamatergic) synaptic terminals on the teleost Mauthner cell known as "Club endings" constitute because of their unusual large size and presence of connexin 35 (Cx35), an ortholog of the widespread mammalian Cx36, a valuable model for the study of electrical transmission. Remarkably, both components of their mixed synaptic response undergo activity-dependent potentiation. Changes in electrical transmission result from interactions with colocalized glutamatergic synapses, the activity of which leads to the activation of Ca(2+)/calmodulin-dependent kinase II (CaMKII), required for the induction of changes in both forms of transmission. However, the distribution of this kinase and potential localization to electrical synapses remains undetermined. Taking advantage of the unparalleled experimental accessibility of Club endings, we explored the presence and intraterminal distribution of CaMKII within these terminals. Here we show that (1) unlike other proteins, both CaMKII labeling and distribution were highly variable between contiguous contacts, and (2) CaMKII was not restricted to the periphery of the terminals, in which glutamatergic synapses are located, but also was present at the center in which gap junctions predominate. Accordingly, double immunolabeling indicated that Cx35 and CaMKII were colocalized, and biochemical analysis showed that these proteins associate. Because CaMKII characteristically undergoes activity-dependent translocation, the observed variability of labeling likely reflects physiological differences between electrical synapses of contiguous Club endings, which remarkably coexist with differing degrees of conductance. Together, our results indicate that CaMKII should be considered a component of electrical synapses, although its association is nonobligatory and likely driven by activity.
Figures

Similar articles
-
Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells.J Neurosci. 2003 Aug 20;23(20):7489-503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. J Neurosci. 2003. PMID: 12930787 Free PMC article.
-
The Roles of Calmodulin and CaMKII in Cx36 Plasticity.Int J Mol Sci. 2021 Apr 25;22(9):4473. doi: 10.3390/ijms22094473. Int J Mol Sci. 2021. PMID: 33922931 Free PMC article. Review.
-
Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain.Neuroscience. 2015 Jan 29;285:166-93. doi: 10.1016/j.neuroscience.2014.10.057. Epub 2014 Nov 4. Neuroscience. 2015. PMID: 25451276 Free PMC article.
-
Dynamics of electrical transmission at club endings on the Mauthner cells.Brain Res Brain Res Rev. 2004 Dec;47(1-3):227-44. doi: 10.1016/j.brainresrev.2004.06.010. Brain Res Brain Res Rev. 2004. PMID: 15572174 Review.
-
Short-range functional interaction between connexin35 and neighboring chemical synapses.Cell Commun Adhes. 2003 Jul-Dec;10(4-6):419-23. doi: 10.1080/15419060390263254. Cell Commun Adhes. 2003. PMID: 14681051 Free PMC article.
Cited by
-
Trafficking of gap junction channels at a vertebrate electrical synapse in vivo.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):E573-82. doi: 10.1073/pnas.1121557109. Epub 2012 Feb 7. Proc Natl Acad Sci U S A. 2012. PMID: 22323580 Free PMC article.
-
Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):4. doi: 10.1186/s12860-016-0120-x. BMC Cell Biol. 2017. PMID: 28124625 Free PMC article. Review.
-
Understanding the Molecular and Cell Biological Mechanisms of Electrical Synapse Formation.Front Neuroanat. 2020 Apr 15;14:12. doi: 10.3389/fnana.2020.00012. eCollection 2020. Front Neuroanat. 2020. PMID: 32372919 Free PMC article.
-
Interaction of acetylcholinesterase with neurexin-1β regulates glutamatergic synaptic stability in hippocampal neurons.Mol Brain. 2014 Mar 5;7:15. doi: 10.1186/1756-6606-7-15. Mol Brain. 2014. PMID: 24594013 Free PMC article.
-
Electrical synapses and their functional interactions with chemical synapses.Nat Rev Neurosci. 2014 Apr;15(4):250-63. doi: 10.1038/nrn3708. Epub 2014 Mar 12. Nat Rev Neurosci. 2014. PMID: 24619342 Free PMC article. Review.
References
-
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–20969. - PMC - PubMed
-
- Bartelmez GW. Mauthner's cell and the nucleus motorius tegmenti. J Comp Neurol. 1915;25:87–128.
-
- Bartelmez GW, Hoerr NL. The vestibular club endings in Ameiurus. Further evidence on the morphology of the synapse. J Comp Neurol. 1933;67:401–428.
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC003186-10/DC/NIDCD NIH HHS/United States
- R01 DC003186-11/DC/NIDCD NIH HHS/United States
- R01 NS052827-04/NS/NINDS NIH HHS/United States
- R56 DC003186/DC/NIDCD NIH HHS/United States
- R01 DC003186-09/DC/NIDCD NIH HHS/United States
- R01 NS052827-03/NS/NINDS NIH HHS/United States
- R56 DC003186-12A2/DC/NIDCD NIH HHS/United States
- P01 DA010044/DA/NIDA NIH HHS/United States
- R01 NS052827/NS/NINDS NIH HHS/United States
- DC03186/DC/NIDCD NIH HHS/United States
- R01 NS052827-02/NS/NINDS NIH HHS/United States
- NS0552827/NS/NINDS NIH HHS/United States
- DA10044/DA/NIDA NIH HHS/United States
- R29 DC003186/DC/NIDCD NIH HHS/United States
- R01 DC003186/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

