Regulation of podocyte BK(Ca) channels by synaptopodin, Rho, and actin microfilaments
- PMID: 20630939
- PMCID: PMC2944294
- DOI: 10.1152/ajprenal.00206.2010
Regulation of podocyte BK(Ca) channels by synaptopodin, Rho, and actin microfilaments
Abstract
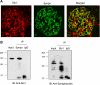
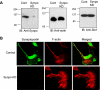
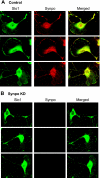
Mechanosensitive large-conductance Ca(2+)-activated K(+) channels encoded by the Slo1 gene (BK(Ca) channels) are expressed in podocytes. Here we show that BK(Ca) channels reciprocally coimmunoprecipitate with synaptopodin (Synpo) in mouse glomeruli, in mouse podocytes, and in a heterologous expression system (HEK293T cells) in which these proteins are transiently expressed. Synpo and Slo1 colocalize along the surface of the glomerular basement membrane in mouse glomeruli. Synpo interacts with BK(Ca) channels at COOH-terminal domains that overlap with an actin-binding domain on the channel molecule that is necessary for trafficking of BK(Ca) channels to the cell surface. Moreover, addition of exogenous beta-actin to mouse podocyte lysates reduces BK(Ca)-Synpo interactions. Coexpression of Synpo increases steady-state surface expression of BK(Ca) channels in HEK293T cells. However, Synpo does not affect the stability of cell surface BK(Ca) channels, suggesting a primary effect on the rate of forward trafficking, and Synpo coexpression does not affect BK(Ca) gating. Conversely, stable knockdown of Synpo expression in mouse podocyte cell lines reduces steady-state surface expression of BK(Ca) channels but does not affect total expression of BK(Ca) channels or their gating. The effects of Synpo on surface expression of BK(Ca) are blocked by inhibition of Rho signaling in HEK293T cells and in podocytes. Functional cell surface BK(Ca) channels in podocytes are also reduced by sustained (2 h) but not acute (15 min) depolymerization of actin with cytochalasin D. Synpo may regulate BK(Ca) channels through its effects on actin dynamics and by modulating interactions between BK(Ca) channels and regulatory proteins of the podocyte slit diaphragm.
Figures



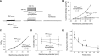
Similar articles
-
Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes.Mol Pharmacol. 2009 Mar;75(3):466-77. doi: 10.1124/mol.108.051912. Epub 2008 Dec 3. Mol Pharmacol. 2009. PMID: 19052171 Free PMC article.
-
A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane.Mol Pharmacol. 2008 Feb;73(2):359-68. doi: 10.1124/mol.107.039743. Epub 2007 Nov 7. Mol Pharmacol. 2008. PMID: 17989352
-
MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface.Am J Physiol Cell Physiol. 2009 Jul;297(1):C55-65. doi: 10.1152/ajpcell.00073.2009. Epub 2009 Apr 29. Am J Physiol Cell Physiol. 2009. PMID: 19403801 Free PMC article.
-
Calcium regulates podocyte actin dynamics.Semin Nephrol. 2012 Jul;32(4):319-26. doi: 10.1016/j.semnephrol.2012.06.003. Semin Nephrol. 2012. PMID: 22958486 Free PMC article. Review.
-
TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology.Am J Physiol Renal Physiol. 2010 Oct;299(4):F689-701. doi: 10.1152/ajprenal.00298.2010. Epub 2010 Aug 4. Am J Physiol Renal Physiol. 2010. PMID: 20685822 Free PMC article. Review.
Cited by
-
Large-Conductance Calcium-Activated Potassium Channels in Glomerulus: From Cell Signal Integration to Disease.Front Physiol. 2016 Jun 21;7:248. doi: 10.3389/fphys.2016.00248. eCollection 2016. Front Physiol. 2016. PMID: 27445840 Free PMC article. Review.
-
Differential expression of the Kv1 voltage-gated potassium channel family in the rat nephron.J Mol Histol. 2014 Oct;45(5):583-97. doi: 10.1007/s10735-014-9581-4. Epub 2014 Jun 20. J Mol Histol. 2014. PMID: 24948003
-
Apolipoprotein-L1 (APOL1): From Sleeping Sickness to Kidney Disease.Cells. 2024 Oct 20;13(20):1738. doi: 10.3390/cells13201738. Cells. 2024. PMID: 39451256 Free PMC article. Review.
-
Mechanism of Regulation of Big-Conductance Ca2+-Activated K+ Channels by mTOR Complex 2 in Podocytes.Front Physiol. 2019 Feb 28;10:167. doi: 10.3389/fphys.2019.00167. eCollection 2019. Front Physiol. 2019. PMID: 30873046 Free PMC article.
-
Upregulated LRRC55 promotes BK channel activation and aggravates cell injury in podocytes.J Exp Med. 2021 Mar 1;218(3):e20192373. doi: 10.1084/jem.20192373. J Exp Med. 2021. PMID: 33346797 Free PMC article.
References
-
- Aktories K, Wilde C, Vogelsgesang M. Rho-modifying C3-like ADP-ribosyltransferases. Rev Physiol Biochem Pharmacol 152: 1–22, 2004 - PubMed
-
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 - PubMed
-
- Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol 289: C49–C57, 2005 - PubMed
-
- Chae KS, Dryer SE. The p38 mitogen-activated protein kinase pathway negatively regulates Ca2+-activated K+ channel trafficking in developing parasympathetic neurons. J Neurochem 94: 367–379, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

