An acyl-covalent enzyme intermediate of lecithin:retinol acyltransferase
- PMID: 20628054
- PMCID: PMC2937952
- DOI: 10.1074/jbc.M110.152314
An acyl-covalent enzyme intermediate of lecithin:retinol acyltransferase
Abstract
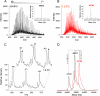
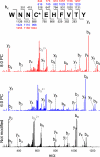
Synthesis of fatty acid retinyl esters determines systemic vitamin A levels and provides substrate for production of visual chromophore (11-cis-retinal) in vertebrates. Lecithin:retinol acyltransferase (LRAT), the main enzyme responsible for retinyl ester formation, catalyzes the transfer of an acyl group from the sn-1 position of phosphatidylcholine to retinol. To delineate the catalytic mechanism of this reaction, we expressed and purified a fully active, soluble form of this enzyme and used it to examine the possible formation of a transient acyl-enzyme intermediate. Detailed mass spectrometry analyses revealed that LRAT undergoes spontaneous, covalent modification upon incubation with a variety of phosphatidylcholine substrates. The addition of an acyl chain occurs at the Cys(161) residue, indicating formation of a thioester intermediate. This observation provides the first direct experimental evidence of thioester intermediate formation that constitutes the initial step in the proposed LRAT catalytic reaction. Additionally, we examined the effect of increasing fatty acyl side chain length in phosphatidylcholine on substrate accessibility in this reaction, which provided insights into the function of the single membrane-spanning domain of LRAT. These observations are critical to understanding the catalytic mechanism of LRAT protein family members as well as other lecithin:acyltransferases wherein Cys residues are required for catalysis.
Figures



Similar articles
-
Topology and membrane association of lecithin: retinol acyltransferase.J Biol Chem. 2007 Jan 19;282(3):2081-90. doi: 10.1074/jbc.M608315200. Epub 2006 Nov 19. J Biol Chem. 2007. PMID: 17114808
-
Acyl CoA:retinol acyltransferase (ARAT) activity is present in bovine retinal pigment epithelium.Exp Eye Res. 2006 Jan;82(1):111-21. doi: 10.1016/j.exer.2005.05.010. Epub 2005 Jul 27. Exp Eye Res. 2006. PMID: 16054134
-
Lecithin:Retinol Acyltransferase: A Key Enzyme Involved in the Retinoid (visual) Cycle.Biochemistry. 2016 Jun 7;55(22):3082-91. doi: 10.1021/acs.biochem.6b00319. Epub 2016 May 23. Biochemistry. 2016. PMID: 27183166 Free PMC article. Review.
-
Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine.J Biol Chem. 1988 Sep 5;263(25):12478-82. J Biol Chem. 1988. PMID: 3410848
-
Acyltransferase families that act on thioesters: Sequences, structures, and mechanisms.Proteins. 2024 Feb;92(2):157-169. doi: 10.1002/prot.26599. Epub 2023 Sep 30. Proteins. 2024. PMID: 37776148 Review.
Cited by
-
Reorganization of cellular retinol-binding protein type 1 and lecithin:retinol acyltransferase during retinyl ester biosynthesis.Biochim Biophys Acta. 2012 Jul;1820(7):859-69. doi: 10.1016/j.bbagen.2012.03.016. Epub 2012 Apr 2. Biochim Biophys Acta. 2012. PMID: 22498138 Free PMC article.
-
Lipoprotein N-Acylation in Staphylococcus aureus Is Catalyzed by a Two-Component Acyl Transferase System.mBio. 2020 Jul 28;11(4):e01619-20. doi: 10.1128/mBio.01619-20. mBio. 2020. PMID: 32723923 Free PMC article.
-
The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Nov;1865(11):158571. doi: 10.1016/j.bbalip.2019.158571. Epub 2019 Nov 23. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31770587 Free PMC article. Review.
-
Allosteric modulation of the substrate specificity of acyl-CoA wax alcohol acyltransferase 2.J Lipid Res. 2017 Apr;58(4):719-730. doi: 10.1194/jlr.M073692. Epub 2017 Jan 17. J Lipid Res. 2017. PMID: 28096191 Free PMC article.
-
Chemistry of the retinoid (visual) cycle.Chem Rev. 2014 Jan 8;114(1):194-232. doi: 10.1021/cr400107q. Epub 2013 Jul 11. Chem Rev. 2014. PMID: 23905688 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

