Gammadelta T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFalpha in mouse models of obesity and metabolic disease
- PMID: 20625397
- PMCID: PMC2896399
- DOI: 10.1371/journal.pone.0011422
Gammadelta T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFalpha in mouse models of obesity and metabolic disease
Abstract
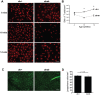
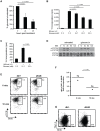
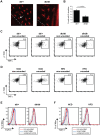
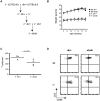
Epithelial cells provide an initial line of defense against damage and pathogens in barrier tissues such as the skin; however this balance is disrupted in obesity and metabolic disease. Skin gammadelta T cells recognize epithelial damage, and release cytokines and growth factors that facilitate wound repair. We report here that hyperglycemia results in impaired skin gammadelta T cell proliferation due to altered STAT5 signaling, ultimately resulting in half the number of gammadelta T cells populating the epidermis. Skin gammadelta T cells that overcome this hyperglycemic state are unresponsive to epithelial cell damage due to chronic inflammatory mediators, including TNFalpha. Cytokine and growth factor production at the site of tissue damage was partially restored by administering neutralizing TNFalpha antibodies in vivo. Thus, metabolic disease negatively impacts homeostasis and functionality of skin gammadelta T cells, rendering host defense mechanisms vulnerable to injury and infection.
Conflict of interest statement
Figures

Similar articles
-
Dysfunctional γδ T cells contribute to impaired keratinocyte homeostasis in mouse models of obesity.J Invest Dermatol. 2011 Dec;131(12):2409-18. doi: 10.1038/jid.2011.241. Epub 2011 Aug 11. J Invest Dermatol. 2011. PMID: 21833015 Free PMC article.
-
Defects in skin gamma delta T cell function contribute to delayed wound repair in rapamycin-treated mice.J Immunol. 2008 Sep 15;181(6):3974-83. doi: 10.4049/jimmunol.181.6.3974. J Immunol. 2008. PMID: 18768852 Free PMC article.
-
Bone marrow mesenchymal stem cells accelerate the hyperglycemic refractory wound healing by inhibiting an excessive inflammatory response.Mol Med Rep. 2017 May;15(5):3239-3244. doi: 10.3892/mmr.2017.6400. Epub 2017 Mar 28. Mol Med Rep. 2017. PMID: 28350113
-
Chronic Inflammation and γδ T Cells.Front Immunol. 2016 May 27;7:210. doi: 10.3389/fimmu.2016.00210. eCollection 2016. Front Immunol. 2016. PMID: 27303404 Free PMC article. Review.
-
Immunomodulation at epithelial sites by obesity and metabolic disease.Immunol Res. 2012 Jun;52(3):182-99. doi: 10.1007/s12026-011-8261-7. Immunol Res. 2012. PMID: 22160809 Review.
Cited by
-
Obesity impairs γδ T cell homeostasis and antiviral function in humans.PLoS One. 2015 Mar 18;10(3):e0120918. doi: 10.1371/journal.pone.0120918. eCollection 2015. PLoS One. 2015. PMID: 25785862 Free PMC article.
-
Functions of skin-resident γδ T cells.Cell Mol Life Sci. 2011 Jul;68(14):2399-408. doi: 10.1007/s00018-011-0702-x. Epub 2011 May 11. Cell Mol Life Sci. 2011. PMID: 21560071 Free PMC article. Review.
-
Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study.Diabetes Obes Metab. 2022 Jan;24(1):160-165. doi: 10.1111/dom.14547. Epub 2021 Oct 1. Diabetes Obes Metab. 2022. PMID: 34494705 Free PMC article. No abstract available.
-
IL-15 Enhances Activation and IGF-1 Production of Dendritic Epidermal T Cells to Promote Wound Healing in Diabetic Mice.Front Immunol. 2017 Nov 24;8:1557. doi: 10.3389/fimmu.2017.01557. eCollection 2017. Front Immunol. 2017. PMID: 29225596 Free PMC article.
-
Weakened IL-15 Production and Impaired mTOR Activation Alter Dendritic Epidermal T Cell Homeostasis in Diabetic Mice.Sci Rep. 2017 Jul 20;7(1):6028. doi: 10.1038/s41598-017-05950-5. Sci Rep. 2017. PMID: 28729536 Free PMC article.
References
-
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, et al. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. - PubMed
-
- Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–131. - PubMed
-
- Komori HK, Meehan TF, Havran WL. Epithelial and mucosal γδ T cells. Curr Opin Immunol. 2006;18:534–538. - PubMed
-
- Xiong N, Raulet DH. Development and selection of γδ T cells. Immunol Rev. 2007;215:15–31. - PubMed
-
- Born WK, Jin N, Aydintug MK, Wands JM, French JD, et al. γδ T lymphocytes-selectable cells within the innate system? J Clin Immunol. 2007;27:133–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

