Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus
- PMID: 20623734
- PMCID: PMC3377327
- DOI: 10.1002/emmm.201000081
Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus
Abstract
Apoptosis is a fundamental host defence mechanism against invading microbes. Inactivation of NF-kappaB attenuates encephalomyocarditis virus (EMCV) virulence by triggering rapid apoptosis of infected cells, thereby pre-emptively limiting viral replication. Recent evidence has shown that hypoxia-inducible factor (HIF) increases NF-kappaB-mediated anti-apoptotic response in clear-cell renal cell carcinoma (CCRCC) that commonly exhibit hyperactivation of HIF due to the loss of its principal negative regulator, von Hippel-Lindau (VHL) tumour suppressor protein. Here, we show that EMCV challenge induces a strong NF-kappaB-dependent gene expression profile concomitant with a lack of interferon-mediated anti-viral response in VHL-null CCRCC, and that multiple established CCRCC cell lines, as well as early-passage primary CCRCC cultured cells, are acutely susceptible to EMCV replication and virulence. Functional restoration of VHL or molecular suppression of HIF or NF-kappaB dramatically reverses CCRCC cellular susceptibility to EMCV-induced killing. Notably, intratumoural EMCV treatment of CCRCC in a murine xenograft model rapidly regresses tumour growth. These findings provide compelling pre-clinical evidence for the usage of EMCV in the treatment of CCRCC and potentially other tumours with elevated HIF/NF-kappaB-survival signature.
Figures
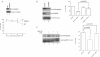
NEMO promotes susceptibility of MEFs to EMCV-induced death. Equal amounts of total cell lysates from WT and NEMO−/− MEFs were immunoblotted with anti-NEMO and anti-Vinculin antibodies (upper panel). WT and NEMO−/− MEFs were infected with EMCV (MOI = 0.1) and viable cells were counted at 2 h intervals post-infection by Annexin V-FITC/propidium iodide staining. Experiments were conducted in triplicate and error bars represent standard errors (lower graph). Two-way ANOVA was applied for statistical analysis between treatments and time points. * and *** denote p < 0.05 and p < 0.001, respectively.
NEMO promotes susceptibility of 786-O CCRCC cells to EMCV-induced death. 786-O cells transfected with NEMO-specific siRNA (siNEMO) or scrambled non-targeting siRNA (siCON) (left panel) were infected with or without EMCV (MOI = 0.1), and viable cells counted 18 h post-infection by Trypan Blue exclusion assay (right graph).
Activated NF-κB pathway promotes susceptibility of 786-O CCRCC cells to EMCV-induced death. 786-O cells were treated with or without JSH-23 (10 µM) in the presence or absence of LPS (10 µg/ml) and the level of nuclear NF-κB visualized by immunoblotting (left panel). 786-O cells treated with or without JSH-23 were challenged with EMCV (MOI = 0.1) and viable cells were counted 18 h post-infection by Trypan Blue exclusion assay (right graph). C1 + C2 denote two splice isoforms of nuclear restricted pre-mRNA binding protein hnRNP. Experiments were performed in triplicate and error bars represent standard errors, independent Student's t-test was used to analyse difference between groups.

Loss of VHL in 786-O cells accentuates NF-κB-mediated transcription upon EMCV challenge. Steady-state 1L8, 1L6 and TNF-α mRNA levels were measured by real-time PCR in 786-O (open bars) and 786-VIIL (solid bars) cells infected with EMCV (MOI = 0.1) for 8 h. Experiments were performed in triplicate and error bars represent standard errors.
Heatmap representation NF-κB-dependent and IFN/IFN-stimulated gene expression in 786-O and 786-VHL cells challenged with EMCV or VSV. Relative gene expression levels of NF-κB-dependent (left panel) and IFN/IFN-stimulated (right panel) genes in 786-O and 786-VHL cells challenged with EMCV or VSV. Actual expression levels as determined by Affymetrix microarray expression analysis arc shown in Tables 1 and 2.
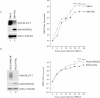
Loss of VHL in 786-O cells enhances EMCV replication. 786-O and 786-VHL cells were lysed and immunoblotted with anti-HA, anti-GLUT-1 and anti-α-Tubulin antibodies (left panel). Cells were challenged with EMCV (MOI = 0.01) and cumulative virus titre was evaluated at 3 h intervals post-infection (right graph). Two-way ANOVA was applied for statistical analysis between treatments and time points. * and *** denote p < 0.05 and p < 0.001, respectively.
Loss of VHL in RCC4 cells enhances EMCV replication. RCC4-MOCK and RCC4-VHL cells were lysed and immunoblotted with anti-HA, anti-GLUT-1 and anti-α-Tubulin antibodies (left panel). Cells were challenged with EMCV (MOI = 0.01) and cumulative virus titre was evaluated at 3 h intervals post-infection (right graph). Two-way ANOVA was applied for statistical analysis between treatments and time points. * and *** denote p < 0.05 and p < 0.001, respectively.

Loss of VHL enhances susceptibility of 786-O cells to EMCV-induced cytotoxicity. 786-O and 786-VHL cells infected with EMCV (MOI = 0.1) were visualized by phase contrast microscopy at various time points (right panel) and viable cells counted at 2 h intervals post-infection by Trypan Blue exclusion assay (left graph). Experiments were conducted in triplicate and error bars represent standard errors. Two-way ANOVA was applied for statistical analysis between treatments and time points. * and *** denote p < 0.05 and p < 0.001, respectively.
VHL-dependent killing by EMCV occurs in a dose-dependent manner. 786-O and 786-VHL cells were infected with twofold serial dilutions of EMCV (starting from MOI = 1) for 24 h. Live cells were stained with 0.05% crystal violet and quantified by integrated density analysis. Nonlinear regression was performed and TCID50 was calculated in GraphPad Prism. Experiments were performed in quadruplicate and error bars represent standard errors.
Visualization of EMCV infection of 786-O cells by light microscopy. 786-O cells uninfected or infected with EMCV (MOI = 0.1) for 7 and 15 h were visualized by electron microscopy. Solid arrow represents virus particle formation in infected cells. Asterisk and open arrows represent lytic cell death. Bars represent 1 µm.
Patient-derived primary CCRCC cells are effectively killed by EMCV. Early passage primary CCRCC cells generated from surgically excised human CCRCC tumours were infected with EMCV (MOI = 0.1 and 1.0) and viable cells were counted at 24 and 48 h post-infection by Trypan Blue exclusion assay. Con denotes uninfected cells. Statistical differences between indicated groups were analysed using the independent Student's t-test.
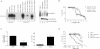
VHL disease-causing mutants Y112H and L188V have varying abilities to regulate the HIF pathway. [35S]methionine-labelled in vitro translated empty plasmid (MOCK), HA-tagged WT or the indicated mutant VHL proteins were mixed with equal amounts of biotinylated HIF1α[ODD-OH] or hydroxylation-defective HIF1α[ODD(PA)] peptides for 30 min at 37°C. Reaction mixtures were then subjected to biotin affinity pull-down (PD) via streptavidin-coated beads and bound radiolabelled HA-VHL proteins were resolved on SDS–PAGE and visualized by autoradiography (left panel). Unmixed radiolabelled in vitro translated HA-tagged WT or the indicated mutant VHL proteins were also immunoprecipitated (IP) with excess anti-HA antibody, resolved on SDS–PAGE and visualized by autoradiography as an indication of equal loading (left graph, lanes 1–4). 786-O, 786-VHL(WT), 786-VHL(Y112H) and 786-VHL(L188V) cells were lysed and immunoblotted with anti-HA, anti-GLUT-1 and anti-α-Tubulin antibodies (right panel).
HIF regulation by VHL influences susceptibility of CCRCC cells to EMCV-induced killing. 786-VHL(Y112H) and 786-VHL(L188V) cells were infected with two-fold serial dilutions of EMCV (starting from MOI = 1) for 24 h. Live cells were stained with 0.05% crystal violet and quantified by integrated density analysis. Nonlinear regression was performed and TCID50 was calculated in GraphPad Prism. Experiments were performed in quadruplicate and error bars represent standard errors.
siRNA-mediated inhibition of HIF2α decreases susceptibility to EMCV-induced death. 786-O cells were transiently transfected with a control scrambled siRNA (siCON) or HIF2α-specific siRNA (siHIF2α). After 24 h, steady-state mRNA levels of HIF2α were measured by real-time qPCR (left graph) and cells were challenged with EMCV (MOI = 0.1). Live cells were quantified by Trypan Blue exclusion assay at 18 h post-infection (right panel). Experiments were performed in triplicate and error bars represent standard errors.
Small molecule inhibitor chetomin-mediated inhibition of HIF2α activity decreases susceptibility to EMCV-induced death. 786-O and 786-VHL cells were treated with or without 50 nM chetomin for 16 h and then challenged with EMCV at the indicated MOI for 18 h. Live cells were stained with 0.05% crystal violet and quantified by integrated density analysis. Experiments were conducted in triplicate and error bars represent standard errors.
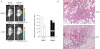
EMCV kills HIF-overexpression tumour cells in vivo. 786-HRE-Luc cells were implanted in dorsal dermis in 6 SCID mice and tumour xenograft visualized 1 day before virus treatment (left panels). Mice were segregated into two groups of 3 where one group received intratumoural injection of live EMCV (bottom panels) while the other group received intratumoural injection of irradiated dead (d) EMCV (top panels), and tumour BLI signals visualized and measured 4 days post-treatment. Average BLI signals from each group measured in photons/seconds/area/steradian are shown with error bars representing standard errors (graph). Representative longitudinal images from the same mouse from each group are also shown (left panels).
EMCV kills tumour cells by necrosis. Haematoxylin and eosin (H&E) staining was performed on the resected xenografts post-termination of treatment. Representative images from each group are shown at 100× magnification. Dashed line, viable/necrotic interface; V, viable cells; N, necrotic cells; a, adipocytes; bv, blood vessels.
Similar articles
-
The von Hippel-Lindau tumor suppressor protein regulates gene expression and tumor growth through histone demethylase JARID1C.Oncogene. 2012 Feb 9;31(6):776-86. doi: 10.1038/onc.2011.266. Epub 2011 Jul 4. Oncogene. 2012. PMID: 21725364 Free PMC article.
-
Suppressive effects of iron chelation in clear cell renal cell carcinoma and their dependency on VHL inactivation.Free Radic Biol Med. 2019 Mar;133:295-309. doi: 10.1016/j.freeradbiomed.2018.12.013. Epub 2018 Dec 13. Free Radic Biol Med. 2019. PMID: 30553971 Free PMC article.
-
Human renal carcinoma cells respond to Newcastle disease virus infection through activation of the p38 MAPK/NF-κB/IκBα pathway.Cell Oncol (Dordr). 2015 Aug;38(4):279-88. doi: 10.1007/s13402-015-0229-5. Epub 2015 May 1. Cell Oncol (Dordr). 2015. PMID: 25930675
-
Significance of PI3K signalling pathway in clear cell renal cell carcinoma in relation to VHL and HIF status.J Clin Pathol. 2021 Apr;74(4):216-222. doi: 10.1136/jclinpath-2020-206693. Epub 2020 May 28. J Clin Pathol. 2021. PMID: 32467322 Review.
-
Von Hippel-Lindau protein signalling in clear cell renal cell carcinoma.Nat Rev Urol. 2024 Nov;21(11):662-675. doi: 10.1038/s41585-024-00876-w. Epub 2024 May 2. Nat Rev Urol. 2024. PMID: 38698165 Review.
Cited by
-
Improved replication efficiency of echovirus 5 after transfection of colon cancer cells using an authentic 5' RNA genome end methodology.Invest New Drugs. 2014 Dec;32(6):1063-70. doi: 10.1007/s10637-014-0136-z. Epub 2014 Jul 23. Invest New Drugs. 2014. PMID: 25052234
-
Innovative strategies in genitourinary cancer: the role of oncolytic viruses.Front Oncol. 2024 Oct 11;14:1461324. doi: 10.3389/fonc.2024.1461324. eCollection 2024. Front Oncol. 2024. PMID: 39464707 Free PMC article. Review.
-
NF-κB inhibition by bortezomib permits IFN-γ-activated RIP1 kinase-dependent necrosis in renal cell carcinoma.Mol Cancer Ther. 2013 Aug;12(8):1568-78. doi: 10.1158/1535-7163.MCT-12-1010. Epub 2013 May 8. Mol Cancer Ther. 2013. PMID: 23657944 Free PMC article.
-
Non-human viruses developed as therapeutic agent for use in humans.Rev Med Virol. 2011 Jul;21(4):227-39. doi: 10.1002/rmv.694. Epub 2011 May 11. Rev Med Virol. 2011. PMID: 21560181 Free PMC article. Review.
-
Efficacy of Systemically Administered Retargeted Oncolytic Herpes Simplex Viruses-Clearance and Biodistribution in Naïve and HSV-Preimmune Mice.Cancers (Basel). 2023 Aug 10;15(16):4042. doi: 10.3390/cancers15164042. Cancers (Basel). 2023. PMID: 37627072 Free PMC article.
References
-
- Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. - PubMed
-
- Algire GH, Legallais FY. Recent developments in the transparent-chamber technique as adapted to the mouse. J Natl Cancer Inst. 1949;10:225–253. incl 228 pl. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Ann Rev Immunol. 1994;12:141–179. - PubMed
-
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

