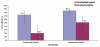Performance of rotavirus vaccines in developed and developing countries
- PMID: 20622508
- PMCID: PMC3322519
- DOI: 10.4161/hv.6.7.11278
Performance of rotavirus vaccines in developed and developing countries
Abstract
The World Health Organization estimates that rotavirus diarrhea results in approximately half a million deaths and approximately 2.4 million hospitalizations in developing countries each year. Two live oral rotavirus vaccines, RotaTeq® (RV 5; Merck) and Rotarix® (RV 1; GlaxoSmithKline) with good efficacy against severe rotavirus disease and a reassuring safety profile could substantially impact the burden of rotavirus disease. In April 2009, WHO provided a recommendation for global introduction of these vaccines in national immunization programs of developing countries worldwide. In this article, we review published data on previous candidate rotavirus vaccines and vaccines in current use, with emphasis on their performance in developed versus developing countries. In developed countries, both first and second generation rotavirus vaccines have demonstrated high efficacy against severe rotavirus disease (pooled efficacy = 73% and 85%, respectively). In developing countries, small early trials for the first generation vaccines failed to provide protection against rotavirus disease (pooled efficacy = 20%), however, trials of the second generation vaccines yielded substantial improvements in efficacy in developing countries (pooled efficacy of 51%), leading to a global recommendation for rotavirus vaccine introduction by WHO. Future efforts for these vaccines should focus on optimizing the efficacy and delivery of these vaccines in challenging target populations of Asia and Africa with the greatest burden of severe rotavirus disease.
Figures
Similar articles
-
Rotavirus infection and the current status of rotavirus vaccines.J Formos Med Assoc. 2012 Apr;111(4):183-93. doi: 10.1016/j.jfma.2011.09.024. Epub 2012 Mar 29. J Formos Med Assoc. 2012. PMID: 22526206 Review.
-
Rotavirus vaccines: targeting the developing world.J Infect Dis. 2005 Sep 1;192 Suppl 1:S160-6. doi: 10.1086/431504. J Infect Dis. 2005. PMID: 16088799
-
Rotarix in developing countries: paving the way for inclusion in national childhood immunization programs in Africa.J Infect Dis. 2010 Sep 1;202 Suppl:S80-6. doi: 10.1086/653547. J Infect Dis. 2010. PMID: 20684722
-
Update on Rotarix: an oral human rotavirus vaccine.Expert Rev Vaccines. 2009 Dec;8(12):1627-41. doi: 10.1586/erv.09.136. Expert Rev Vaccines. 2009. PMID: 19943758 Review.
-
Rotavirus vaccines: opportunities and challenges.Hum Vaccin. 2009 Feb;5(2):57-69. doi: 10.4161/hv.5.2.6924. Epub 2009 Feb 8. Hum Vaccin. 2009. PMID: 18838873 Review.
Cited by
-
Pathogen surveillance in the informal settlement, Kibera, Kenya, using a metagenomics approach.PLoS One. 2019 Oct 10;14(10):e0222531. doi: 10.1371/journal.pone.0222531. eCollection 2019. PLoS One. 2019. PMID: 31600207 Free PMC article.
-
Mouse Subcutaneous BCG Vaccination and Mycobacterium tuberculosis Infection Alter the Lung and Gut Microbiota.Microbiol Spectr. 2022 Jun 29;10(3):e0169321. doi: 10.1128/spectrum.01693-21. Epub 2022 Jun 2. Microbiol Spectr. 2022. PMID: 35652642 Free PMC article.
-
Immunogenicity of RV1 and RV5 vaccines administered in standard and interchangeable mixed schedules: a randomized, double-blind, non-inferiority clinical trial in Mexican infants.Front Public Health. 2024 Feb 23;12:1356932. doi: 10.3389/fpubh.2024.1356932. eCollection 2024. Front Public Health. 2024. PMID: 38463163 Free PMC article. Clinical Trial.
-
Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta-analysis of observational studies.BMC Infect Dis. 2017 Aug 15;17(1):569. doi: 10.1186/s12879-017-2613-4. BMC Infect Dis. 2017. PMID: 28810833 Free PMC article.
-
Evaluation of the Influence of Gastrointestinal Coinfections on Rotavirus Vaccine Effectiveness in Botswana.Pediatr Infect Dis J. 2018 Mar;37(3):e58-e62. doi: 10.1097/INF.0000000000001828. Pediatr Infect Dis J. 2018. PMID: 29189612 Free PMC article.
References
-
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:9–15. - PubMed
-
- Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous