Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans
- PMID: 20620666
- PMCID: PMC2927200
- DOI: 10.1016/j.neurobiolaging.2010.04.033
Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans
Abstract
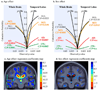
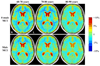
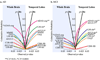
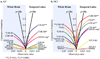
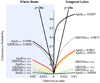
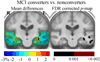
We set out to determine factors that influence the rate of brain atrophy in 1-year longitudinal magnetic resonance imaging (MRI) data. With tensor-based morphometry (TBM), we mapped the 3-dimensional profile of progressive atrophy in 144 subjects with probable Alzheimer's disease (AD) (age: 76.5 +/- 7.4 years), 338 with amnestic mild cognitive impairment (MCI; 76.0 +/- 7.2), and 202 healthy controls (77.0 +/- 5.1), scanned twice, 1 year apart. Statistical maps revealed significant age and sex differences in atrophic rates. Brain atrophic rates were about 1%-1.5% faster in women than men. Atrophy was faster in younger than older subjects, most prominently in mild cognitive impairment, with a 1% increase in the rates of atrophy and 2% in ventricular expansion, for every 10-year decrease in age. TBM-derived atrophic rates correlated with reduced beta-amyloid and elevated tau levels (n = 363) at baseline, baseline and progressive deterioration in clinical measures, and increasing numbers of risk alleles for the ApoE4 gene. TBM is a sensitive, high-throughput biomarker for tracking disease progression in large imaging studies; sub-analyses focusing on women or younger subjects gave improved sample size requirements for clinical trials.
2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Optimizing power to track brain degeneration in Alzheimer's disease and mild cognitive impairment with tensor-based morphometry: an ADNI study of 515 subjects.Neuroimage. 2009 Dec;48(4):668-81. doi: 10.1016/j.neuroimage.2009.07.011. Epub 2009 Jul 14. Neuroimage. 2009. PMID: 19615450 Free PMC article.
-
MRI-based brain atrophy rates in ADNI phase 2: acceleration and enrichment considerations for clinical trials.Neurobiol Aging. 2016 Jan;37:26-37. doi: 10.1016/j.neurobiolaging.2015.09.018. Epub 2015 Oct 19. Neurobiol Aging. 2016. PMID: 26545631 Free PMC article.
-
Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort.Neurobiol Aging. 2010 Aug;31(8):1401-18. doi: 10.1016/j.neurobiolaging.2010.04.029. Neurobiol Aging. 2010. PMID: 20620664 Free PMC article.
-
Mapping Alzheimer's disease progression in 1309 MRI scans: power estimates for different inter-scan intervals.Neuroimage. 2010 May 15;51(1):63-75. doi: 10.1016/j.neuroimage.2010.01.104. Epub 2010 Feb 6. Neuroimage. 2010. PMID: 20139010 Free PMC article.
-
Tracking Alzheimer's disease.Ann N Y Acad Sci. 2007 Feb;1097:183-214. doi: 10.1196/annals.1379.017. Ann N Y Acad Sci. 2007. PMID: 17413023 Free PMC article. Review.
Cited by
-
Sex-specific DNA methylation differences in Alzheimer's disease pathology.Acta Neuropathol Commun. 2021 Apr 26;9(1):77. doi: 10.1186/s40478-021-01177-8. Acta Neuropathol Commun. 2021. PMID: 33902726 Free PMC article.
-
The rise of large-scale imaging studies in psychiatry.Gigascience. 2014 Nov 25;3:29. doi: 10.1186/2047-217X-3-29. eCollection 2014. Gigascience. 2014. PMID: 25793106 Free PMC article. Review.
-
DeepResBat: deep residual batch harmonization accounting for covariate distribution differences.bioRxiv [Preprint]. 2024 Aug 6:2024.01.18.574145. doi: 10.1101/2024.01.18.574145. bioRxiv. 2024. Update in: Med Image Anal. 2025 Jan;99:103354. doi: 10.1016/j.media.2024.103354 PMID: 38293022 Free PMC article. Updated. Preprint.
-
Early Onset of Sex-Dependent Mitochondrial Deficits in the Cortex of 3xTg Alzheimer's Mice.Cells. 2020 Jun 24;9(6):1541. doi: 10.3390/cells9061541. Cells. 2020. PMID: 32599904 Free PMC article.
-
Molecular insights into sex-specific metabolic alterations in Alzheimer's mouse brain using multi-omics approach.Alzheimers Res Ther. 2023 Jan 9;15(1):8. doi: 10.1186/s13195-023-01162-4. Alzheimers Res Ther. 2023. PMID: 36624525 Free PMC article.
References
-
- Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging. 1998;19(2):109–116. - PubMed
-
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. - PubMed
-
- Ashburner J, Friston KJ. Human Brain Function. Academic Press; 2003. Morphometry.
-
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Zhu W, Wang L, Yuan Y, Qian Y. Absent gender differences of hippocampal atrophy in amnestic type mild cognitive impairment. Neurosci Lett. 2009;450(2):85–89. - PubMed
-
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14(2):298–309. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG020098-07/AG/NIA NIH HHS/United States
- R01 EB007813/EB/NIBIB NIH HHS/United States
- P30 AG010129-19/AG/NIA NIH HHS/United States
- R01 EB008432-02/EB/NIBIB NIH HHS/United States
- U54 RR021813-05/RR/NCRR NIH HHS/United States
- R01 EB008432-01A2/EB/NIBIB NIH HHS/United States
- R01 AG020098-09/AG/NIA NIH HHS/United States
- U54 RR021813/RR/NCRR NIH HHS/United States
- R01 AG020098/AG/NIA NIH HHS/United States
- K01 AG030514-02/AG/NIA NIH HHS/United States
- R01 EB007813-04/EB/NIBIB NIH HHS/United States
- U54 RR021813-020001/RR/NCRR NIH HHS/United States
- R01 EB007813-03/EB/NIBIB NIH HHS/United States
- K01 AG030514/AG/NIA NIH HHS/United States
- K01 AG030514-04/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- U54-RR021813/RR/NCRR NIH HHS/United States
- R01 EB008281-14/EB/NIBIB NIH HHS/United States
- R01 HD050735/HD/NICHD NIH HHS/United States
- R01 EB008432/EB/NIBIB NIH HHS/United States
- R01 HD050735-03/HD/NICHD NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- R01 EB008281-13/EB/NIBIB NIH HHS/United States
- R01 EB008281/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

