Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria
- PMID: 20618959
- PMCID: PMC2912831
- DOI: 10.1186/1471-2172-11-37
Differential and coordinated expression of defensins and cytokines by gingival epithelial cells and dendritic cells in response to oral bacteria
Abstract
Background: Epithelial cells and dendritic cells (DCs) both initiate and contribute to innate immune responses to bacteria. However, much less is known about the coordinated regulation of innate immune responses between GECs and immune cells, particularly DCs in the oral cavity. The present study was conducted to investigate whether their responses are coordinated and are bacteria-specific in the oral cavity.
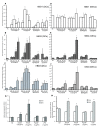
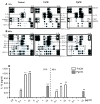
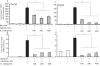
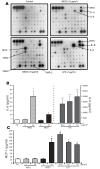
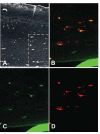
Results: The beta-defensin antimicrobial peptides hBD1, hBD2 and hBD3 were expressed by immature DCs as well as gingival epithelial cells (GECs). HBD1, hBD2 and hBD3 are upregulated in DCs while hBD2 and hBD3 are upregulated in GECs in response to bacterial stimulation. Responses of both cell types were bacteria-specific, as demonstrated by distinctive profiles of hBDs mRNA expression and secreted cytokines and chemokines in response to cell wall preparations of various bacteria of different pathogenicity: Fusobacterium nucleatum, Actinomyces naeslundii and Porphyromonas gingivalis. The regulation of expression of hBD2, IL-8, CXCL2/GRObeta and CCL-20/MIP3alpha by GECs was greatly enhanced by conditioned medium from bacterially activated DCs. This enhancement was primarily mediated via IL-1beta, since induction was largely attenuated by IL-1 receptor antagonist. In addition, the defensins influence DCs by eliciting differential cytokine and chemokine secretion. HBD2 significantly induced IL-6, while hBD3 induced MCP-1 to approximately the same extent as LPS, suggesting a unique role in immune responses.
Conclusions: The results suggest that cytokines, chemokines and beta-defensins are involved in interaction of these two cell types, and the responses are bacteria-specific. Differential and coordinated regulation between GECs and DCs may be important in regulation of innate immune homeostasis and response to pathogens in the oral cavity.
Figures
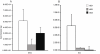
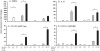
Similar articles
-
Activation of protective responses in oral epithelial cells by Fusobacterium nucleatum and human beta-defensin-2.J Med Microbiol. 2007 Jul;56(Pt 7):976-987. doi: 10.1099/jmm.0.47198-0. J Med Microbiol. 2007. PMID: 17577065
-
Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier.Infect Immun. 2000 May;68(5):2907-15. doi: 10.1128/IAI.68.5.2907-2915.2000. Infect Immun. 2000. PMID: 10768988 Free PMC article.
-
Actinobacillus actinomycetemcomitans outer membrane protein 100 triggers innate immunity and production of beta-defensin and the 18-kilodalton cationic antimicrobial protein through the fibronectin-integrin pathway in human gingival epithelial cells.Infect Immun. 2006 Sep;74(9):5211-20. doi: 10.1128/IAI.00056-06. Infect Immun. 2006. PMID: 16926414 Free PMC article.
-
Species-specific regulation of innate immunity by vitamin D signaling.J Steroid Biochem Mol Biol. 2016 Nov;164:246-253. doi: 10.1016/j.jsbmb.2015.09.016. Epub 2015 Sep 11. J Steroid Biochem Mol Biol. 2016. PMID: 26369615 Review.
-
Differential expression of human beta defensin 2 and 3 in gastric mucosa of Helicobacter pylori-infected individuals.Helicobacter. 2013 Feb;18(1):6-12. doi: 10.1111/hel.12000. Epub 2012 Aug 28. Helicobacter. 2013. PMID: 23067102 Review.
Cited by
-
Understanding the roles of gingival beta-defensins.J Oral Microbiol. 2012;4. doi: 10.3402/jom.v4i0.15127. Epub 2012 Feb 28. J Oral Microbiol. 2012. PMID: 22389759 Free PMC article.
-
Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells.Sci Rep. 2019 Oct 31;9(1):15779. doi: 10.1038/s41598-019-52115-7. Sci Rep. 2019. PMID: 31673005 Free PMC article.
-
Towards the Application of Human Defensins as Antivirals.Biomol Ther (Seoul). 2018 May 1;26(3):242-254. doi: 10.4062/biomolther.2017.172. Biomol Ther (Seoul). 2018. PMID: 29310427 Free PMC article. Review.
-
The concentrations of IL-8 and IL-6 in gingival crevicular fluid during nickel-chromium alloy porcelain crown restoration.J Mater Sci Mater Med. 2013 Jul;24(7):1717-22. doi: 10.1007/s10856-013-4924-3. Epub 2013 Apr 7. J Mater Sci Mater Med. 2013. PMID: 23564008 Clinical Trial.
-
Histone deacetylase-mediated regulation of the antimicrobial peptide hBD2 differs in intestinal cell lines and cultured tissue.Sci Rep. 2018 Aug 27;8(1):12886. doi: 10.1038/s41598-018-31125-x. Sci Rep. 2018. PMID: 30150730 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

