Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells
- PMID: 20616344
- PMCID: PMC2913362
- DOI: 10.2353/ajpath.2010.091012
Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells
Abstract
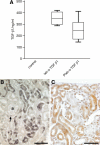
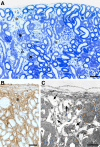
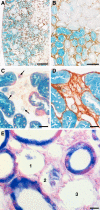
We recently showed in a tetracycline-controlled transgenic mouse model that overexpression of transforming growth factor (TGF)-beta1 in renal tubules induces widespread peritubular fibrosis and focal degeneration of nephrons. In the present study we have analyzed the mechanisms underlying these phenomena. The initial response to tubular cell-derived TGF-beta1 consisted of a robust proliferation of peritubular cells and deposition of collagen. On sustained expression, nephrons degenerated in a focal pattern. This process started with tubular dedifferentiation and proceeded to total decomposition of tubular cells by autophagy. The final outcome was empty collapsed remnants of tubular basement membrane embedded into a dense collagenous fibrous tissue. The corresponding glomeruli survived as atubular remnants. Thus, TGF-beta1 driven autophagy may represent a novel mechanism of tubular decomposition. The fibrosis seen in between intact tubules and in areas of tubular decomposition resulted from myofibroblasts that were derived from local fibroblasts. No evidence was found for a transition of tubular cells into myofibroblasts. Neither tracing of injured tubules in electron micrographs nor genetic tagging of tubular epithelial cells revealed cells transgressing the tubular basement membrane. In conclusion, overexpression of TGF-beta1 in renal tubules in vivo induces interstitial proliferation, tubular autophagy, and fibrosis, but not epithelial-to-mesenchymal transition.
Figures




Similar articles
-
Autophagy activates EGR1 via MAPK/ERK to induce FGF2 in renal tubular cells for fibroblast activation and fibrosis during maladaptive kidney repair.Autophagy. 2024 May;20(5):1032-1053. doi: 10.1080/15548627.2023.2281156. Epub 2023 Nov 18. Autophagy. 2024. PMID: 37978868 Free PMC article.
-
Micro-vesicles from mesenchymal stem cells over-expressing miR-34a inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition in renal tubular epithelial cells in vitro.Chin Med J (Engl). 2020 Apr 5;133(7):800-807. doi: 10.1097/CM9.0000000000000720. Chin Med J (Engl). 2020. PMID: 32149762 Free PMC article.
-
Nerve growth factor exposure promotes tubular epithelial-mesenchymal transition via TGF-β1 signaling activation.Growth Factors. 2015;33(3):169-80. doi: 10.3109/08977194.2015.1054989. Epub 2015 Jun 11. Growth Factors. 2015. PMID: 26066770
-
The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy.Cytokine Growth Factor Rev. 2011 Jun;22(3):131-9. doi: 10.1016/j.cytogfr.2011.06.002. Epub 2011 Jul 14. Cytokine Growth Factor Rev. 2011. PMID: 21757394 Review.
-
The role of epithelial-to-mesenchymal transition in renal fibrosis.J Mol Med (Berl). 2004 Mar;82(3):175-81. doi: 10.1007/s00109-003-0517-9. Epub 2004 Jan 30. J Mol Med (Berl). 2004. PMID: 14752606 Review.
Cited by
-
Plasticity of renal erythropoietin-producing cells governs fibrosis.J Am Soc Nephrol. 2013 Oct;24(10):1599-616. doi: 10.1681/ASN.2013010030. Epub 2013 Jul 5. J Am Soc Nephrol. 2013. PMID: 23833259 Free PMC article.
-
Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction.Autophagy. 2016 Jun 2;12(6):976-98. doi: 10.1080/15548627.2016.1166317. Epub 2016 Apr 28. Autophagy. 2016. PMID: 27123926 Free PMC article.
-
Autophagy in kidney health and disease.Antioxid Redox Signal. 2014 Jan 20;20(3):519-37. doi: 10.1089/ars.2013.5363. Epub 2013 Jun 28. Antioxid Redox Signal. 2014. PMID: 23642034 Free PMC article. Review.
-
Tubular Overexpression of Angiopoietin-1 Attenuates Renal Fibrosis.PLoS One. 2016 Jul 25;11(7):e0158908. doi: 10.1371/journal.pone.0158908. eCollection 2016. PLoS One. 2016. PMID: 27454431 Free PMC article.
-
Cdc42-interacting protein-4 promotes TGF-Β1-induced epithelial-mesenchymal transition and extracellular matrix deposition in renal proximal tubular epithelial cells.Int J Biol Sci. 2012;8(6):859-69. doi: 10.7150/ijbs.3490. Epub 2012 Jun 13. Int J Biol Sci. 2012. PMID: 22745576 Free PMC article.
References
-
- Fan J, Ng Y, Hill P, Nikolic-Paterson D, Mu W, Atkins R, Lan H. Transforming growth factor-beta regulates tubular epithelial myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. - PubMed
-
- Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. - PubMed
-
- Zeisberg E, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med. 2004;82:175–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

