A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription
- PMID: 20610623
- PMCID: PMC2912498
- DOI: 10.1158/0008-5472.CAN-10-1028
A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription
Abstract
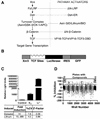
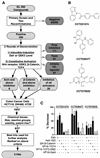
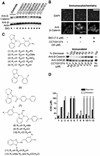
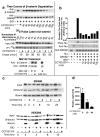
The Wnt signaling pathway is frequently deregulated in cancer due to mutations in genes encoding APC, beta-catenin, and axin. To identify small-molecule inhibitors of Wnt signaling as potential therapeutics, a diverse chemical library was screened using a transcription factor reporter cell line in which the activity of the pathway was induced at the level of Disheveled protein. A series of deconvolution studies was used to focus on three compound series that selectively killed cancer cell lines with constitutive Wnt signaling. Activities of the compounds included the ability to induce degradation of beta-catenin that had been stabilized by a glycogen synthase kinase-3 (GSK-3) inhibitor. This screen illustrates a practical approach to identify small-molecule inhibitors of Wnt signaling that can seed the development of agents suitable to treat patients with Wnt-dependent tumors.
(c)2010 AACR.
Figures
Similar articles
-
An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway.Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):5954-63. doi: 10.1073/pnas.1017496108. Epub 2011 Mar 10. Proc Natl Acad Sci U S A. 2011. PMID: 21393571 Free PMC article.
-
Winning WNT: race to Wnt signaling inhibitors.Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):5929-30. doi: 10.1073/pnas.1103102108. Epub 2011 Mar 30. Proc Natl Acad Sci U S A. 2011. PMID: 21451134 Free PMC article. No abstract available.
-
Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α.Nat Chem Biol. 2010 Nov;6(11):829-36. doi: 10.1038/nchembio.453. Epub 2010 Oct 3. Nat Chem Biol. 2010. PMID: 20890287 Free PMC article.
-
Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: inhibitors of the Wnt/beta-catenin signaling pathway as novel anticancer drugs.J Pharmacol Sci. 2009 Feb;109(2):179-83. doi: 10.1254/jphs.08r28fm. Epub 2009 Jan 29. J Pharmacol Sci. 2009. PMID: 19179804 Review.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
-
Investigation of 3-aryl-pyrimido[5,4-e][1,2,4]triazine-5,7-diones as small molecule antagonists of β-catenin/TCF transcription.Bioorg Med Chem Lett. 2013 Nov 1;23(21):5814-20. doi: 10.1016/j.bmcl.2013.08.111. Epub 2013 Sep 7. Bioorg Med Chem Lett. 2013. PMID: 24060489 Free PMC article.
-
Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities.Mol Cell Biochem. 2021 Sep;476(9):3513-3536. doi: 10.1007/s11010-021-04174-6. Epub 2021 May 17. Mol Cell Biochem. 2021. PMID: 33999334 Free PMC article. Review.
-
Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish.PLoS Genet. 2015 Jul 2;11(7):e1005305. doi: 10.1371/journal.pgen.1005305. eCollection 2015 Jul. PLoS Genet. 2015. PMID: 26134322 Free PMC article.
-
Pharmacological modulation of beta-catenin and its applications in cancer therapy.J Cell Mol Med. 2013 Apr;17(4):449-56. doi: 10.1111/jcmm.12033. Epub 2013 Mar 14. J Cell Mol Med. 2013. PMID: 23490077 Free PMC article. Review.
-
Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells.Aging Cell. 2019 Feb;18(1):e12844. doi: 10.1111/acel.12844. Epub 2018 Dec 12. Aging Cell. 2019. PMID: 30548452 Free PMC article.
References
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. - PubMed
-
- Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17:499–508. - PubMed
-
- Ewan KB, Dale TC. The potential for targeting oncogenic WNT/beta-catenin signaling in therapy. Current drug targets. 2008;9:532–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

