Intrinsically disordered proteins are potential drug targets
- PMID: 20598937
- PMCID: PMC2918680
- DOI: 10.1016/j.cbpa.2010.06.169
Intrinsically disordered proteins are potential drug targets
Abstract
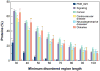
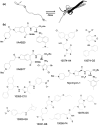
Intrinsically disordered (ID) proteins that lack stable secondary and tertiary structure in substantial regions (or throughout) are prevalent in eukaryotes. They exist as ensembles of rapidly fluctuating structures and many undergo coupled folding and binding reactions. Because ID proteins are overrepresented in major disease pathways they are desirable targets for inhibition; however, the feasibility of targeting proteins without defined structures was unclear. Recently, small molecules have been found that bind to the disordered regions of c-Myc, Abeta, EWS-Fli1, and various peptides. As with structured targets, initial hits were further optimized to increase specificity and affinity. Given the number and biological importance of ID proteins, the ability to inhibit their interactions opens tremendous potential in chemical biology and drug discovery.
2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Drugs for 'protein clouds': targeting intrinsically disordered transcription factors.Curr Opin Pharmacol. 2010 Dec;10(6):782-8. doi: 10.1016/j.coph.2010.09.005. Curr Opin Pharmacol. 2010. PMID: 20889377
-
Why does the silica-binding protein "Si-tag" bind strongly to silica surfaces? Implications of conformational adaptation of the intrinsically disordered polypeptide to solid surfaces.Colloids Surf B Biointerfaces. 2011 Sep 1;86(2):359-63. doi: 10.1016/j.colsurfb.2011.04.020. Epub 2011 Apr 21. Colloids Surf B Biointerfaces. 2011. PMID: 21592750
-
Intrinsically disordered proteins and novel strategies for drug discovery.Expert Opin Drug Discov. 2012 Jun;7(6):475-88. doi: 10.1517/17460441.2012.686489. Epub 2012 May 5. Expert Opin Drug Discov. 2012. PMID: 22559227 Review.
-
Understanding protein non-folding.Biochim Biophys Acta. 2010 Jun;1804(6):1231-64. doi: 10.1016/j.bbapap.2010.01.017. Epub 2010 Feb 1. Biochim Biophys Acta. 2010. PMID: 20117254 Free PMC article. Review.
-
How to design a drug for the disordered proteins?Drug Discov Today. 2013 Oct;18(19-20):910-5. doi: 10.1016/j.drudis.2013.04.008. Epub 2013 Apr 30. Drug Discov Today. 2013. PMID: 23643489 Review.
Cited by
-
Structure-Based Small Molecule Modulation of a Pre-Amyloid State: Pharmacological Enhancement of IAPP Membrane-Binding and Toxicity.Biochemistry. 2015 Jun 9;54(22):3555-64. doi: 10.1021/acs.biochem.5b00052. Epub 2015 May 22. Biochemistry. 2015. PMID: 25966003 Free PMC article.
-
Uncovering the role of the flexible C-terminal tail: A model study with Strep-tagged GFP.Biochim Open. 2015 Nov 30;2:1-8. doi: 10.1016/j.biopen.2015.11.004. eCollection 2016 Jun. Biochim Open. 2015. PMID: 29632832 Free PMC article.
-
Visualizing single-molecule conformational transition and binding dynamics of intrinsically disordered proteins.Nat Commun. 2023 Aug 25;14(1):5203. doi: 10.1038/s41467-023-41018-x. Nat Commun. 2023. PMID: 37626077 Free PMC article.
-
Mechanism of the interaction between the intrinsically disordered C-terminus of the pro-apoptotic ARTS protein and the Bir3 domain of XIAP.PLoS One. 2011;6(9):e24655. doi: 10.1371/journal.pone.0024655. Epub 2011 Sep 20. PLoS One. 2011. PMID: 21949740 Free PMC article.
-
Classification of Complete Proteomes of Different Organisms and Protein Sets Based on Their Protein Distributions in Terms of Some Key Attributes of Proteins.Int J Genomics. 2018 Mar 4;2018:9784161. doi: 10.1155/2018/9784161. eCollection 2018. Int J Genomics. 2018. PMID: 29686995 Free PMC article.
References
-
- Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5:821–834. - PubMed
-
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. - PubMed
-
- Yin H, Hamilton AD. Strategies for targeting protein-protein interactions with synthetic agents. Angew Chem-Int Edit. 2005;44:4130–4163. - PubMed
-
- Clackson T, Wells JA. A Hot-Spot of Binding-Energy in a Hormone-Receptor Interface. Science. 1995;267:383–386. - PubMed
-
- Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18:756–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

