Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease
- PMID: 20593505
- PMCID: PMC2896757
- DOI: 10.3748/wjg.v16.i25.3187
Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease
Abstract
Aim: To evaluate the relationship between thiopurine S-methyltransferase (TPMT) polymorphisms and thiopurine-induced adverse drug reactions (ADRs) in inflammatory bowel disease (IBD).
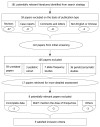
Methods: Eligible articles that compared the frequency of TPMT polymorphisms among thiopurine-tolerant and -intolerant adult IBD patients were included. Statistical analysis was performed with Review Manager 5.0. Sub-analysis/sensitivity analysis was also performed.
Results: Nine studies that investigated a total of 1309 participants met our inclusion criteria. The incidence of TPMT gene mutation was increased 2.93-fold (95% CI: 1.68-5.09, P = 0.0001) and 5.93-fold (95% CI: 2.96-11.88, P < 0.00001), respectively, in IBD patients with thiopurine-induced overall ADRs and bone marrow toxicity (BMT), compared with controls. The OR for TPMT gene mutation in IBD patients with thiopurine-induced hepatotoxicity and pancreatitis was 1.51 (95% CI: 0.54-4.19, P = 0.43) and 1.02 (95% CI: 0.26-3.99, P = 0.98) vs controls, respectively.
Conclusion: This meta-analysis suggests that the TPMT polymorphisms are associated with thiopurine-induced overall ADRs and BMT, but not with hepatotoxicity and pancreatitis.
Figures


Similar articles
-
Association between thiopurine S-methyltransferase polymorphisms and thiopurine-induced adverse drug reactions in patients with inflammatory bowel disease: a meta-analysis.PLoS One. 2015 Mar 23;10(3):e0121745. doi: 10.1371/journal.pone.0121745. eCollection 2015. PLoS One. 2015. PMID: 25799415 Free PMC article.
-
The impact of thiopurine-S-methyltransferase genotype on the adverse drug reactions to azathioprine in patients with inflammatory bowel diseases.Bratisl Lek Listy. 2013;114(4):199-205. doi: 10.4149/bll_2013_042. Bratisl Lek Listy. 2013. PMID: 23514552
-
Association between Thiopurine S-Methyltransferase Polymorphisms and Azathioprine-Induced Adverse Drug Reactions in Patients with Autoimmune Diseases: A Meta-Analysis.PLoS One. 2015 Dec 3;10(12):e0144234. doi: 10.1371/journal.pone.0144234. eCollection 2015. PLoS One. 2015. PMID: 26633017 Free PMC article.
-
Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease.J Gastroenterol Hepatol. 2005 Aug;20(8):1149-57. doi: 10.1111/j.1440-1746.2005.03832.x. J Gastroenterol Hepatol. 2005. PMID: 16048561 Review.
-
Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing.Clin Pharmacokinet. 2007;46(3):187-208. doi: 10.2165/00003088-200746030-00001. Clin Pharmacokinet. 2007. PMID: 17328579 Review.
Cited by
-
Thiopurine S-Methyltransferase as a Pharmacogenetic Biomarker: Significance of Testing and Review of Major Methods.Cardiovasc Hematol Agents Med Chem. 2017 Nov 8;15(1):23-30. doi: 10.2174/1871525715666170529091921. Cardiovasc Hematol Agents Med Chem. 2017. PMID: 28552060 Free PMC article. Review.
-
Thiopurines in the Management of Crohn's Disease: Safety and Efficacy Profile in Patients with Normal TPMT Activity-A Retrospective Study.Can J Gastroenterol Hepatol. 2016;2016:1034834. doi: 10.1155/2016/1034834. Epub 2016 Mar 29. Can J Gastroenterol Hepatol. 2016. PMID: 27446822 Free PMC article.
-
Does cardiology hold pharmacogenetics to an inconsistent standard? A comparison of evidence among recommendations.Pharmacogenomics. 2018 Oct;19(15):1203-1216. doi: 10.2217/pgs-2018-0097. Epub 2018 Sep 10. Pharmacogenomics. 2018. PMID: 30196751 Free PMC article.
-
Liver involvement in inflammatory bowel disease: What should the clinician know?World J Hepatol. 2021 Nov 27;13(11):1534-1551. doi: 10.4254/wjh.v13.i11.1534. World J Hepatol. 2021. PMID: 34904028 Free PMC article. Review.
-
Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease.World J Gastroenterol. 2011 Oct 7;17(37):4166-73. doi: 10.3748/wjg.v17.i37.4166. World J Gastroenterol. 2011. PMID: 22072847 Free PMC article. Review.
References
-
- Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009:CD000067. - PubMed
-
- Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2009:CD000545. - PubMed
-
- Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, Relling MV, Evans WE. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. - PubMed
-
- Winter JW, Gaffney D, Shapiro D, Spooner RJ, Marinaki AM, Sanderson JD, Mills PR. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069–1077. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

