2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion
- PMID: 20593179
- PMCID: PMC3093301
- DOI: 10.1007/s00280-010-1391-0
2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion
Abstract
Purpose: The glucose analog and glycolytic inhibitor 2-deoxy-D-glucose (2-DG), which is currently under clinical evaluation for targeting cancer cells, not only blocks glycolysis thereby reducing cellular ATP, but also interferes with N-linked glycosylation, which leads to endoplasmic reticulum (ER) stress and an unfolded protein response (UPR). Both bioenergetic challenge and ER stress have been shown to activate autophagy, a bulk cellular degradation process that plays either a pro- or anti-death role. Here, we investigate which pathway 2-DG interferes with that activates autophagy and the role of this process in modulating 2-DG-induced toxicity.
Methods: Pancreatic cancer cell line 1420, melanoma cell line MDA-MB-435 and breast cancer cell line SKBR3 were used to investigate the relationship between induction by 2-DG treatment of ER stress/UPR, ATP reduction and activation of autophagy. ER stress/UPR (Grp78 and CHOP) and autophagy (LC3B II) markers were assayed by immunoblotting, while ATP levels were measured using the CellTiter-Glo Luminescent Cell Viability Assay. Autophagy was also measured by immunofluorescence utilizing LC3B antibody. Cell death was detected with a Vi-Cell cell viability analyzer using trypan blue exclusion.
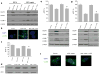
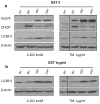
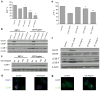
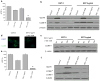
Results: In the three different cancer cell lines described earlier, we find that 2-DG upregulates autophagy, increases ER stress and lowers ATP levels. Addition of exogenous mannose reverses 2-DG-induced autophagy and ER stress but does not recover the lowered levels of ATP. Moreover, under anaerobic conditions where 2-DG severely depletes ATP, autophagy is diminished rather than activated, which correlates with lowered levels of the ER stress marker Grp78. Additionally, when autophagy is blocked by siRNA, cell sensitivity to 2-DG is increased corresponding with upregulation of ER stress-mediated apoptosis. Similar increased toxicity is observed with 3-methyladenine, a known autophagy inhibitor. In contrast, rapamycin which enhances autophagy reduces 2-DG-induced toxicity.
Conclusions: Overall, these results indicate that the major mechanism by which 2-DG stimulates autophagy is through ER stress/UPR and not by lowering ATP levels. Furthermore, autophagy plays a protective role against 2-DG-elicited cell death apparently by relieving ER stress. These data suggest that combining autophagy inhibitors with 2-DG may be useful clinically.
Conflict of interest statement
Figures


Similar articles
-
Endoplasmic reticulum stress induced by 2-deoxyglucose but not glucose starvation activates AMPK through CaMKKβ leading to autophagy.Biochem Pharmacol. 2013 May 15;85(10):1463-77. doi: 10.1016/j.bcp.2013.02.037. Epub 2013 Mar 13. Biochem Pharmacol. 2013. PMID: 23500541
-
2-Deoxy-D-glucose has distinct and cell line-specific effects on the survival of different cancer cells upon antitumor drug treatment.FEBS J. 2018 Dec;285(24):4590-4601. doi: 10.1111/febs.14687. Epub 2018 Nov 17. FEBS J. 2018. PMID: 30375744
-
Inhibition of endoplasmic-reticulum-stress-mediated autophagy enhances the effectiveness of chemotherapeutics on pancreatic cancer.J Transl Med. 2018 Jul 9;16(1):190. doi: 10.1186/s12967-018-1562-z. J Transl Med. 2018. PMID: 29986726 Free PMC article.
-
Oncogenic BRAF, endoplasmic reticulum stress, and autophagy: Crosstalk and therapeutic targets in cutaneous melanoma.Mutat Res Rev Mutat Res. 2020 Jul-Sep;785:108321. doi: 10.1016/j.mrrev.2020.108321. Epub 2020 Jul 7. Mutat Res Rev Mutat Res. 2020. PMID: 32800272 Review.
-
Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy?Int J Mol Sci. 2023 Jan 13;24(2):1566. doi: 10.3390/ijms24021566. Int J Mol Sci. 2023. PMID: 36675080 Free PMC article. Review.
Cited by
-
Calcium homeostasis and ER stress in control of autophagy in cancer cells.Biomed Res Int. 2015;2015:352794. doi: 10.1155/2015/352794. Epub 2015 Mar 3. Biomed Res Int. 2015. PMID: 25821797 Free PMC article. Review.
-
PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation.Nat Commun. 2016 Oct 25;7:13280. doi: 10.1038/ncomms13280. Nat Commun. 2016. PMID: 27779186 Free PMC article.
-
2-Deoxy-d-glucose Suppresses the In Vivo Antitumor Efficacy of Erlotinib in Head and Neck Squamous Cell Carcinoma Cells.Oncol Res. 2016;24(1):55-64. doi: 10.3727/096504016X14586627440192. Oncol Res. 2016. PMID: 27178822 Free PMC article.
-
Endoplasmic reticulum stress signaling in mammalian oocytes and embryos: life in balance.Int Rev Cell Mol Biol. 2015;316:227-65. doi: 10.1016/bs.ircmb.2015.01.005. Epub 2015 Feb 20. Int Rev Cell Mol Biol. 2015. PMID: 25805126 Free PMC article. Review.
-
2-Deoxy glucose regulate MMP-9 in a SIRT-1 dependent and NFkB independent mechanism.Mol Cell Biochem. 2016 Dec;423(1-2):197-206. doi: 10.1007/s11010-016-2837-4. Epub 2016 Oct 4. Mol Cell Biochem. 2016. PMID: 27704463
References
-
- Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224:963–969. - PubMed
-
- Chen W, Gueron M. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie. 1992;74:867–873. - PubMed
-
- Liu H, Hu YP, Savaraj N, Priebe W, Lampidis TJ. Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry. 2001;40:5542–5547. - PubMed
-
- Liu H, Savaraj N, Priebe W, Lampidis TJ. Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: a strategy for solid tumor therapy (model C) Biochem Pharmacol. 2002;64:1745–1751. - PubMed
-
- Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

