The clinically approved proteasome inhibitor PS-341 efficiently blocks influenza A virus and vesicular stomatitis virus propagation by establishing an antiviral state
- PMID: 20592098
- PMCID: PMC2937650
- DOI: 10.1128/JVI.00533-10
The clinically approved proteasome inhibitor PS-341 efficiently blocks influenza A virus and vesicular stomatitis virus propagation by establishing an antiviral state
Abstract
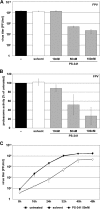
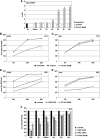
Recently it has been shown that the proinflammatory NF-kappaB pathway promotes efficient influenza virus propagation. Based on these findings, it was suggested that NF-kappaB blockade may be a promising approach for antiviral intervention. The classical virus-induced activation of the NF-kappaB pathway requires proteasomal degradation of the inhibitor of NF-kappaB, IkappaB. Therefore, we hypothesized that inhibition of proteasomal IkappaB degradation should impair influenza A virus (IAV) replication. We chose the specific proteasome inhibitor PS-341, which is a clinically approved anticancer drug also known as Bortezomib or Velcade. As expected, PS-341 treatment of infected A549 cells in a concentration range that was not toxic resulted in a significant reduction of progeny virus titers. However, we could not observe the proposed suppression of NF-kappaB-signaling in vitro. Rather, PS-341 treatment resulted in an induction of IkappaB degradation and activation of NF-kappaB as well as the JNK/AP-1 pathway. This coincides with enhanced expression of antiviral genes, such as interleukin-6 and, most importantly, MxA, which is a strong interferon (IFN)-induced suppressor of influenza virus replication. This suggests that PS-341 may act as an antiviral agent via induction of the type I IFN response. Accordingly, PS-341 did not affect virus titers in Vero cells, which lack type I IFN genes, but strongly inhibited replication of vesicular stomatitis virus (VSV), a highly IFN-sensitive pathogen. Thus, we conclude that PS-341 blocks IAV and VSV replication by inducing an antiviral state mediated by the NF-kappaB-dependent expression of antivirus-acting gene products.
Figures





Similar articles
-
Proteasome inhibitor PS-341 (bortezomib) induces calpain-dependent IkappaB(alpha) degradation.J Biol Chem. 2010 May 21;285(21):16096-104. doi: 10.1074/jbc.M109.072694. Epub 2010 Mar 24. J Biol Chem. 2010. PMID: 20335171 Free PMC article.
-
Oncolytic vesicular stomatitis virus and bortezomib are antagonistic against myeloma cells in vitro but have additive anti-myeloma activity in vivo.Exp Hematol. 2013 Dec;41(12):1038-49. doi: 10.1016/j.exphem.2013.09.005. Epub 2013 Sep 22. Exp Hematol. 2013. PMID: 24067362 Free PMC article.
-
Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition.Cancer Res. 2001 May 1;61(9):3535-40. Cancer Res. 2001. PMID: 11325813
-
Proteasome inhibition in cancer: development of PS-341.Semin Oncol. 2001 Dec;28(6):613-9. doi: 10.1016/s0093-7754(01)90034-x. Semin Oncol. 2001. PMID: 11740819 Review.
-
The role of nuclear factor-kappaB in the biology and treatment of multiple myeloma.Semin Oncol. 2001 Dec;28(6):626-33. doi: 10.1016/s0093-7754(01)90036-3. Semin Oncol. 2001. PMID: 11740821 Review.
Cited by
-
A Mass Spectrometry-Based Profiling of Interactomes of Viral DDB1- and Cullin Ubiquitin Ligase-Binding Proteins Reveals NF-κB Inhibitory Activity of the HIV-2-Encoded Vpx.Front Immunol. 2018 Dec 19;9:2978. doi: 10.3389/fimmu.2018.02978. eCollection 2018. Front Immunol. 2018. PMID: 30619335 Free PMC article.
-
Proteomic Signature of Host Response to SARS-CoV-2 Infection in the Nasopharynx.Mol Cell Proteomics. 2021;20:100134. doi: 10.1016/j.mcpro.2021.100134. Epub 2021 Aug 14. Mol Cell Proteomics. 2021. PMID: 34400346 Free PMC article.
-
Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects.Clin Cancer Res. 2014 Jul 15;20(14):3787-98. doi: 10.1158/1078-0432.CCR-14-0553. Epub 2014 May 9. Clin Cancer Res. 2014. PMID: 24815720 Free PMC article.
-
Proteasome Inhibitor PS-341 Effectively Blocks Infection by the Severe Fever with Thrombocytopenia Syndrome Virus.Virol Sin. 2019 Oct;34(5):572-582. doi: 10.1007/s12250-019-00162-9. Epub 2019 Oct 21. Virol Sin. 2019. PMID: 31637631 Free PMC article.
-
Combining Oncolytic Viruses and Small Molecule Therapeutics: Mutual Benefits.Cancers (Basel). 2021 Jul 6;13(14):3386. doi: 10.3390/cancers13143386. Cancers (Basel). 2021. PMID: 34298601 Free PMC article. Review.
References
-
- Adams, J., M. Behnke, S. Chen, A. A. Cruickshank, L. R. Dick, L. Grenier, J. M. Klunder, Y. T. Ma, L. Plamondon, and R. L. Stein. 1998. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg. Med. Chem. Lett. 8:333-338. - PubMed
-
- Adams, J., V. J. Palombella, E. A. Sausville, J. Johnson, A. Destree, D. D. Lazarus, J. Maas, C. S. Pien, S. Prakash, and P. J. Elliott. 1999. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59:2615-2622. - PubMed
-
- An, J., Y. Sun, M. Fisher, and M. B. Rettig. 2004. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-κB dependent. Mol. Cancer Ther. 3:727-736. - PubMed
-
- Baumeister, W., J. Walz, F. Zuhl, and E. Seemuller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367-380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

