The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells
- PMID: 20579400
- PMCID: PMC2917038
- DOI: 10.1186/bcr2598
The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells
Abstract
Introduction: Microtubule-associated protein tau (MAPT) inhibits the function of taxanes and high expression of MAPT decreases the sensitivity to taxanes. The relationship between estrogen receptor (ER) and MAPT in breast cancer is unclear. In this study, we examined the correlation of MAPT expression with the sensitivity of human breast cancer cells to taxanes, and the relationship between ER and MAPT.
Methods: The correlation between MAPT expression and sensitivity to taxanes was investigated in 12 human breast cancer cell lines. Alterations in cellular sensitivity to taxanes were evaluated after knockdown of MAPT expression. ER expression was knocked down or stimulated in MAPT- and ER-positive cell lines to examine the relationship between ER and MAPT. The cells were also treated with hormone drugs (tamoxifen and fulvestrant) and taxanes.
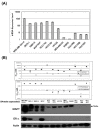
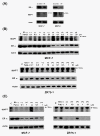
Results: mRNA expression of MAPT did not correlate with sensitivity to taxanes. However, expression of MAPT protein isoforms of less than 70 kDa was correlated with a low sensitivity to taxanes. Downregulation of MAPT increased cellular sensitivity to taxanes. MAPT protein expression was increased by stimulation with 17-beta-estradiol or tamoxifen, but decreased by ER downregulation and by fulvestrant, an ER inhibitor. The combination of fulvestrant with taxanes had a synergistic effect, whereas tamoxifen and taxanes had an antagonistic effect.
Conclusions: Expression of MAPT protein isoforms of less than 70 kDa is correlated with a low sensitivity to taxanes in breast cancer cells. ER influences MAPT expression and fulvestrant increases the sensitivity to taxanes in MAPT- and ER-positive breast cancer cells.
Figures


Similar articles
-
Combination treatment with fulvestrant and various cytotoxic agents (doxorubicin, paclitaxel, docetaxel, vinorelbine, and 5-fluorouracil) has a synergistic effect in estrogen receptor-positive breast cancer.Cancer Sci. 2011 Nov;102(11):2038-42. doi: 10.1111/j.1349-7006.2011.02050.x. Epub 2011 Sep 1. Cancer Sci. 2011. PMID: 21801281
-
Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin.Breast Cancer Res. 2010;12(3):R32. doi: 10.1186/bcr2586. Epub 2010 Jun 4. Breast Cancer Res. 2010. PMID: 20525379 Free PMC article.
-
Effects of fulvestrant alone or combined with different steroids in human breast cancer cells in vitro.Climacteric. 2008 Aug;11(4):315-21. doi: 10.1080/13697130802232500. Climacteric. 2008. PMID: 18645697
-
The use of selective estrogen receptor modulators and selective estrogen receptor down-regulators in breast cancer.Best Pract Res Clin Endocrinol Metab. 2004 Mar;18(1):47-66. doi: 10.1016/j.beem.2003.08.002. Best Pract Res Clin Endocrinol Metab. 2004. PMID: 14687597 Review.
-
Fulvestrant: a review of its use in hormone receptor-positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy.Drugs. 2004;64(6):633-48. doi: 10.2165/00003495-200464060-00009. Drugs. 2004. PMID: 15018596 Review.
Cited by
-
Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress.Nat Neurosci. 2024 Oct;27(10):1918-1933. doi: 10.1038/s41593-024-01740-1. Epub 2024 Aug 26. Nat Neurosci. 2024. PMID: 39187706
-
MAPT (Tau) expression is a biomarker for an increased rate of survival in pediatric neuroblastoma.Cell Cycle. 2018;17(21-22):2474-2483. doi: 10.1080/15384101.2018.1542898. Epub 2018 Nov 18. Cell Cycle. 2018. PMID: 30394813 Free PMC article.
-
Expression of reactive species related genes is associated with patient survival in luminal B breast cancer.Free Radic Biol Med. 2018 May 20;120:170-180. doi: 10.1016/j.freeradbiomed.2018.03.011. Epub 2018 Mar 12. Free Radic Biol Med. 2018. PMID: 29545070 Free PMC article.
-
Clinical relevance of DNA microarray analyses using archival formalin-fixed paraffin-embedded breast cancer specimens.BMC Cancer. 2011 Jun 16;11:253:1-13. doi: 10.1186/1471-2407-11-253. BMC Cancer. 2011. PMID: 21679412 Free PMC article.
-
Estrogen receptor-α is localized to neurofibrillary tangles in Alzheimer's disease.Sci Rep. 2016 Feb 3;6:20352. doi: 10.1038/srep20352. Sci Rep. 2016. PMID: 26837465 Free PMC article.
References
-
- Piccart-Gebhart MJ, Burzykowski T, Buyse M, Sledge G, Carmichael J, Lück HJ, Mackey JR, Nabholtz JM, Paridaens R, Biganzoli L, Jassem J, Bontenbal M, Bonneterre J, Chan S, Basaran GA, Therasse P. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–1986. doi: 10.1200/JCO.2007.10.8399. - DOI - PubMed
-
- Mazouni C, Kau SW, Frye D, Andre F, Kuerer HM, Buchholz TA, Symmans WF, Anderson K, Hess KR, Gonzalez-Angulo AM, Hortobagyi GN, Buzdar AU, Pusztai L. Inclusion of taxanes, particularly weekly paclitaxel, in preoperative chemotherapy improves pathologic complete response rate in estrogen receptor-positive breast cancers. Ann Oncol. 2007;18:874–880. doi: 10.1093/annonc/mdm008. - DOI - PubMed
-
- McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. - PubMed
-
- Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, Ayers M, Ross JS, Zhang P, Buchholz TA, Kuerer H, Green M, Arun B, Hortobagyi GN, Symmans WF, Pusztai L. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

