Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions
- PMID: 20579074
- PMCID: PMC2951498
- DOI: 10.1111/j.1750-3639.2010.00413.x
Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions
Erratum in
- Brain Pathol. 2010 Nov;20(6):1119
Abstract
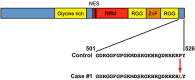
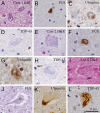
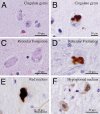
Juvenile amyotrophic lateral sclerosis (ALS) with basophilic inclusions is a well-recognized entity. However, the molecular underpinnings of this devastating disease are poorly understood. Here, we present genetic and neuropathological characterizations in two young women with fatal rapidly progressive ALS with basophilic inclusions. In one case, a germline mutation (P525L) was detected in the fused in sarcoma/translocated in liposarcoma (FUS/TLS) gene, whereas no mutation was identified in the other case. Postmortem examination in both cases revealed severe loss of spinal motor neurons with remaining neurons showing basophilic inclusions that contain abnormal aggregates of FUS proteins and disorganized intracellular organelles, including mitochondria and endoplasmic reticulum. In both patients, the FUS-positive inclusions were also detected in neurons in layers IV-V of cerebral cortex and several brainstem nuclei. In contrast, spinal motor neurons in patients with late-onset sporadic ALS showed no evidence of abnormal accumulation of FUS protein. These results underscore the importance of FUS mutations and pathology in rapidly progressive juvenile ALS. Furthermore, our study represents the first detailed characterizations of neuropathological findings in rapidly progressive juvenile ALS patients with a mutation in the FUS/TLS gene.
Brain Pathology © 2010 International Society of Neuropathology. No claim to original US government works.
Figures


Similar articles
-
Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations.Neurology. 2010 Aug 17;75(7):611-8. doi: 10.1212/WNL.0b013e3181ed9cde. Epub 2010 Jul 28. Neurology. 2010. PMID: 20668261 Free PMC article.
-
An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions.Neuropathology. 2011 Feb;31(1):71-6. doi: 10.1111/j.1440-1789.2010.01129.x. Neuropathology. 2011. PMID: 20573033
-
FUS/TLS-immunoreactive neuronal and glial cell inclusions increase with disease duration in familial amyotrophic lateral sclerosis with an R521C FUS/TLS mutation.J Neuropathol Exp Neurol. 2012 Sep;71(9):779-88. doi: 10.1097/NEN.0b013e318264f164. J Neuropathol Exp Neurol. 2012. PMID: 22878663
-
[Amyotrophic lateral sclerosis (ALS) with the mutations in the fused in sarcoma/translocated in liposarcoma gene].Rinsho Shinkeigaku. 2013;53(11):1080-3. doi: 10.5692/clinicalneurol.53.1080. Rinsho Shinkeigaku. 2013. PMID: 24291885 Review. Japanese.
-
TDP-43 and FUS/TLS: sending a complex message about messenger RNA in amyotrophic lateral sclerosis?FEBS J. 2011 Oct;278(19):3569-77. doi: 10.1111/j.1742-4658.2011.08277.x. Epub 2011 Sep 6. FEBS J. 2011. PMID: 21810174 Review.
Cited by
-
Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS.Front Cell Neurosci. 2015 Feb 17;9:41. doi: 10.3389/fncel.2015.00041. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25741238 Free PMC article. Review.
-
Molecular motor proteins and amyotrophic lateral sclerosis.Int J Mol Sci. 2011;12(12):9057-82. doi: 10.3390/ijms12129057. Epub 2011 Dec 7. Int J Mol Sci. 2011. PMID: 22272119 Free PMC article. Review.
-
RNA-Binding Proteins in Amyotrophic Lateral Sclerosis.Mol Cells. 2018 Sep 30;41(9):818-829. doi: 10.14348/molcells.2018.0243. Epub 2018 Aug 29. Mol Cells. 2018. PMID: 30157547 Free PMC article. Review.
-
Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis.Neuron. 2013 Aug 7;79(3):416-38. doi: 10.1016/j.neuron.2013.07.033. Neuron. 2013. PMID: 23931993 Free PMC article. Review.
-
Expression of human FUS protein in Drosophila leads to progressive neurodegeneration.Protein Cell. 2011 Jun;2(6):477-86. doi: 10.1007/s13238-011-1065-7. Epub 2011 Jul 12. Protein Cell. 2011. PMID: 21748598 Free PMC article.
References
-
- Aizawa H, Kimura T, Hashimoto K, Yahara O, Okamoto K, Kikuchi K (2000) Basophilic cytoplasmic inclusions in a case of sporadic juvenile amyotrophic lateral sclerosis. J Neurol Sci 176:109–113. - PubMed
-
- Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J et al (2010) FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 81:639–645. - PubMed
-
- Boillee S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52:39–59. - PubMed
-
- Dion PA, Daoud H, Rouleau GA (2009) Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet 10:769–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

